Figures & data
Table 1. Baseline characteristics of patients with Herpes labialis.
Table 2. Vaccine-related reactions.
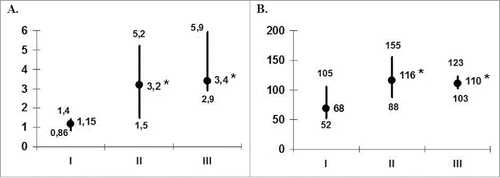
Figure 1. Frequency of recurrences (A) and the duration of interrecurrent period (B) in the patients with herpes labialis during DC-vaccination. The data are presented as median and interquartile range. The numbers of recurrences per month (A) and duration of interrecurrent period (B) were analyzed in 14 patients before (I), at the end of vaccination (II) and during the first 6 months of the follow-up period (III). *pU < 0.05 and **pU < 0.01 - the significance of differences with baseline values (U - nonparametric Mann-Whitney test).
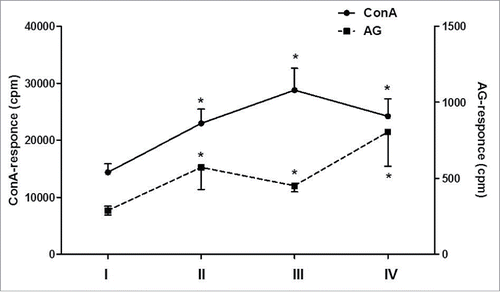
Table 3. Long-term outcomes of DC vaccination estimated by interviewing the patients with herpes labialis followed for over a year.
Figure 2. Antigen and mitogen reactivity of patients' MNCs (n = 14) during DC-vaccination. The data are presented as stimulation indexes (IS; median and interquartile range) of HSV1gD (A) and ConA (B) on the proliferative response of peripheral blood MNC from 14 patients with herpes labialis assessed before treatment (I), at the end of vaccination (II) and during the first 6 months of the follow-up period (III). *pU < 0.05 - the significance of differences with baseline values (U - nonparametric Mann-Whitney test).
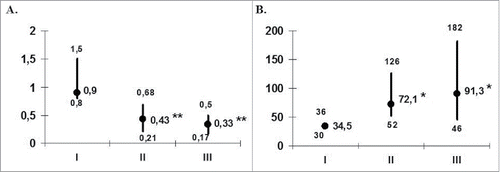
Figure 3. Proliferative response of patients' MNCs (n = 7) to mitogen or antigen stimulation during DC vaccination and 48-month follow-up period. The proliferation of patients' MNC to mitogen (ConA; the left vertical axis) or viral antigen (HSV1gD; the right vertical axis) was analyzed in 7 patients with herpes labialis before DC therapy (I), at the end of vaccination (II), 6 months (III) and 48 months (IV) after vaccination. Data are shown as M ± SE (cpm). *pW < 0.05 - the significance of differences with baseline values (W - non-parametric Wilcoxon test for paired samples).