Figures & data
Figure 1. (A) J8-specific or (B) DT-specific serum IgG in SWISS mice (n = 5 female and n = 5 male mice) administered J8-DT/alum, DT/alum or PBS/alum with 2 or 3 intramuscular immunizations on days 0, 21 and 42 (x3 immunisations only). Mice were immunized as previously describedCitation30 with minor modifications. Briefly, 4–6 week old SWISS mice (n = 5 female and n = 5 male/group housed separately) were immunized intramuscularly on day 0 with J8-DT (50 ug), DT (50 ug) or PBS formulated with alum (Alhydrogel 2%). Selected cohorts were boosted on day 21 or on days 21 and 42. Sera from the cohorts were collected on selected days pre and post-immunisation. SWISS mice were purchased from The Animal Resource Centre, Western Australia. Statistical significance was calculated using GraphPad Prism version 6. One-way Analysis of Variance (ANOVA) was applied to evaluate significant differences in absorbance levels. The Dunnett's test was performed as a post-hoc test. A P value of < 0.05 was considered significant. Data was expressed as mean ± SEM.
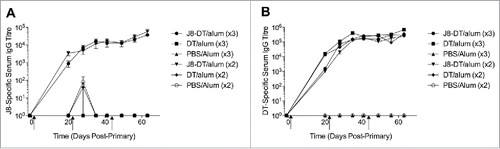
Figure 2. Immunogenicity and protective efficacy of J8-DT/alum in BALB/c mice. (A) Cohorts of BALB/c mice (4–6 weeks old) were subcutaneously immunised with 50 ug of J8-DT vaccine formulation on days 0, 21 and 28. The J8-specific IgG titers on days 20, 27 and 35 are shown. Efficacy of the vaccine formulation following bacterial skin challenge with 88/30 GAS strain for cohorts of mice that received either 1, 2 or 3 doses of vaccine and the control cohort administered PBS plus adjuvant. Skin (B and C) and blood (D and E) bioburden was determined for days 3 and 6 post-exposure respectively. Mean (+/− SEM) shown. A P value of <0.05 was considered significant. Statistical significance was calculated using GraphPad Prism version 6.
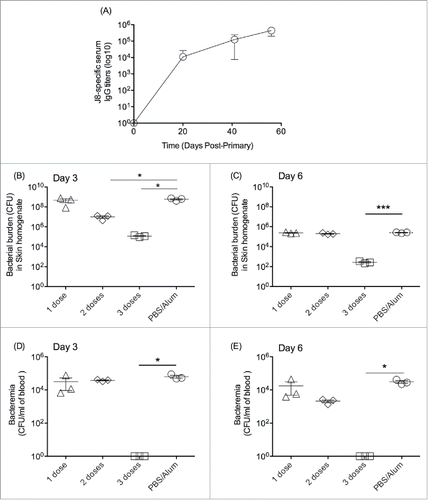
Table 1. In vitro opsonisation (mean %+/−SEM) of the GAS strain 88/30.
Figure 3. Immunogenicity of the vaccine formulation (J8-DT/alum) in New Zealand White rabbits. Rabbits were administered intramuscularly 50 ug of J8-DT/alum vaccine formulation on days 0, 21 and 42. J8-specific serum IgG titers were determined on the days indicated using a similar protocol used for the mice studies. New Zealand White Rabbits were sourced by Veterinary Institute, South Australia.
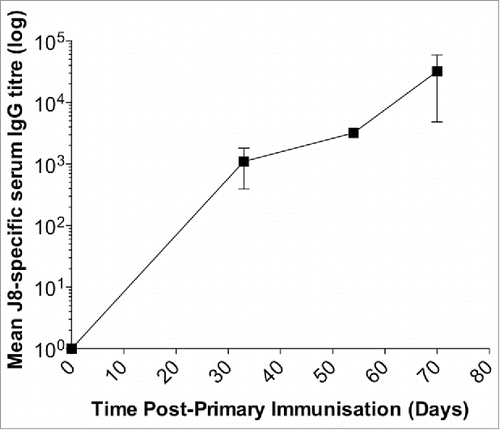
Figure 4. Antibody responses to rM5 and J8-peptide in immunized Lewis rats. IgG antibody reactivity of serum from Lewis rats immunized with rM5 (•), J8-DT (▪), DT (▴) or PBS (negative control) (▾), to rM5 (A) or J8-peptide (B). All rats received a primary immunization on day 0, followed by an intraperitoneal injection of B. pertussis on day 1 and 3. A booster was administered on day 7. Absorbance values of rat sera at 1:100 dilution are shown, with mean absorbance value presented as horizontal lines. Significance was determined by ANOVA with the post hoc Dunnet's test (*, P < 0.01; **, P < 0.001). Lewis rats were purchased from The Animal Resource Centre, Western Australia.
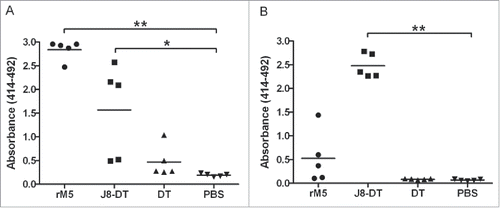
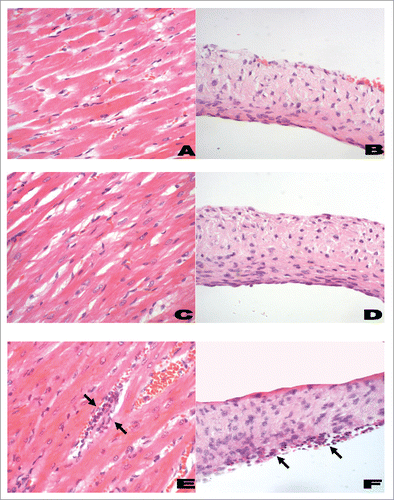
Figure 5. Histological features of myocardium (left panel) and heart valves (right panel) from Lewis rats. A representative histological section from rats immunized with PBS and adjuvant (negative control; A and B) had little or no infiltration of inflammatory cells into the myocardium or valvular tissue. Rats immunized with J8-DT (C and D) had minimal changes comparable to the negative control rats. However, in rM5-immunized rats (positive control; E and F) there was evidence of mononuclear cell infiltration (Arrows) in the myocardial and valvular tissue (H&E; original magnifications: A, C and E, x200; B, D and F, x400). The rat hearts were excised and fixed in 10% neutral buffered formalin for 48 h prior to being embedded in paraffin. Tissue samples were cut in 5 µm-thick sections on a microtome and stained with hematoxylin and eosin (H&E) using standard procedures. Sections were examined by a pathologist who was blinded to the treatment groups, using a light microscope fitted with a QImaging camera. Evidence of inflammatory changes and cellular infiltration in the myocardium and mitral, aortic and tricuspid valve leaflets were assessed.