Figures & data
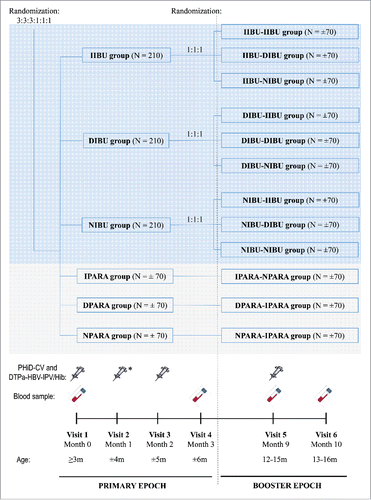
Figure 1. Participant flow chart. Footnote: Primary vaccination: PHiD-CV and DTPa-(HBV)-IPV/Hib at 3, 4, and 5 months of age, with the following prophylactic antipyretic regimen: IIBU, immediate ibuprofen; DIBU, delayed ibuprofen; NIBU, no ibuprofen; IPARA, immediate paracetamol; DPARA, delayed paracetamol; NPARA, no paracetamol. Booster vaccination: PHiD-CV and DTPa-HBV-IPV/Hib at 12–15 months of age, with the following prophylactic antipyretic regimen: at primary vaccination: immediate ibuprofen, and at booster: immediate (IIBU-IIBU), delayed (IIBU-DIBU) or no ibuprofen (IIBU-NIBU); at primary vaccination: delayed ibuprofen, and at booster: immediate (DIBU-IIBU), delayed (DIBU-DIBU) or no ibuprofen (DIBU-NIBU); at primary vaccination: no ibuprofen, and at booster: immediate (NIBU-IIBU), delayed (NIBU-DIBU) or no ibuprofen (NIBU-NIBU); immediate paracetamol at primary vaccination and no paracetamol at booster (IPARA-NPARA); delayed paracetamol at primary vaccination and immediate paracetamol at booster (DPARA-IPARA); no paracetamol at primary vaccination, and immediate paracetamol at booster (NPARA-IPARA). ATP, according-to-protocol; N, number of participants.
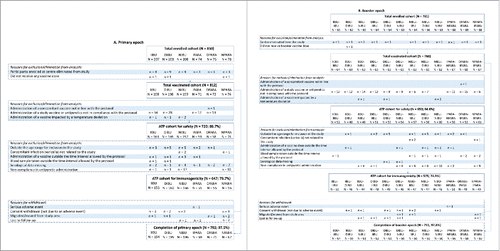
Table 1. Serotype-specific pneumococcal and protein D antibody responses with pairwise group comparisons for the ibuprofen groups, at one month post-dose three (ATP cohort for immunogenicity).
Table 2. Exploratory analysis: serotype-specific pneumococcal and protein D antibody responses with pairwise group comparisons for the paracetamol groups, one month post-dose three (ATP cohort for immunogenicity).
Table 3. Exploratory analysis: antibody responses to DTPa-HBV-IPV/Hib antigens with pairwise group comparisons, one month post-dose three (ATP cohort for immunogenicity).
Figure 2. Incidence of fever post-primary (A) and post-booster (B) vaccination (TVC). Footnote: Primary vaccination: PHiD-CV and DTPa-(HBV)-IPV/Hib at 3, 4, and 5 months of age, with the following prophylactic antipyretic regimen: IIBU, immediate ibuprofen; DIBU, delayed ibuprofen; NIBU, no ibuprofen; IPARA, immediate paracetamol; DPARA, delayed paracetamol; NPARA, no paracetamol. Booster vaccination: PHiD-CV and DTPa-HBV-IPV/Hib at 12–15 months of age, with the following prophylactic antipyretic regimen: at primary vaccination: immediate ibuprofen, and at booster: immediate (IIBU-IIBU), delayed (IIBU-DIBU) or no ibuprofen (IIBU-NIBU); at primary vaccination: delayed ibuprofen, and at booster: immediate (DIBU-IIBU), delayed (DIBU-DIBU) or no ibuprofen (DIBU-NIBU); at primary vaccination: no ibuprofen, and at booster: immediate (NIBU-IIBU), delayed (NIBU-DIBU) or no ibuprofen (NIBU-NIBU); immediate paracetamol at primary vaccination and no paracetamol at booster (IPARA-NPARA); delayed paracetamol at primary vaccination and immediate paracetamol at booster (DPARA-IPARA); no paracetamol at primary vaccination, and immediate paracetamol at booster (NPARA-IPARA). Fever: rectal temperature ≥38.0°C; Grade 3 fever: rectal temperature >40°C or axillary/oral/tympanic temperature >39.5°C; TVC, total vaccinated cohort. Error bars indicate 95% confidence intervals.
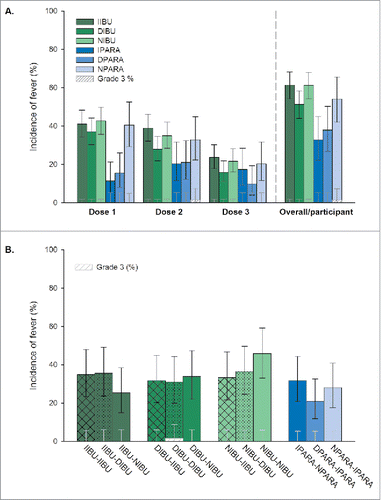
Figure 3. Study design. Footnote: Primary vaccination: PHiD-CV and DTPa-(HBV)-IPV/Hib at 3, 4, and 5 months of age, with the following prophylactic antipyretic regimen: IIBU, immediate ibuprofen; DIBU, delayed ibuprofen; NIBU, no ibuprofen; IPARA, immediate paracetamol; DPARA, delayed paracetamol; NPARA, no paracetamol. Booster vaccination: PHiD-CV and DTPa-HBV-IPV/Hib at 12–15 months of age, with the following prophylactic antipyretic regimen: at primary vaccination: immediate ibuprofen, and at booster: immediate (IIBU-IIBU), delayed (IIBU-DIBU) or no ibuprofen (IIBU-NIBU); at primary vaccination: delayed ibuprofen, and at booster: immediate (DIBU-IIBU), delayed (DIBU-DIBU) or no ibuprofen (DIBU-NIBU); at primary vaccination: no ibuprofen, and at booster: immediate (NIBU-IIBU), delayed (NIBU-DIBU) or no ibuprofen (NIBU-NIBU); immediate paracetamol at primary vaccination and no paracetamol at booster (IPARA-NPARA); delayed paracetamol at primary vaccination and immediate paracetamol at booster (DPARA-IPARA); no paracetamol at primary vaccination, and immediate paracetamol at booster (NPARA-IPARA). N, number of children per group; m, months; * DTPa-IPV/Hib instead of DTPa-HBV-IPV/Hib.