Figures & data
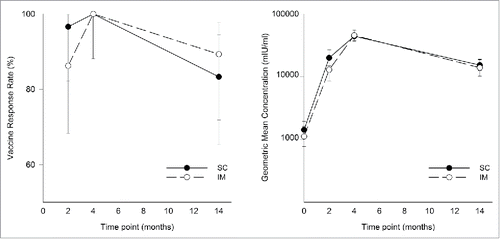
Figure 2. Vaccine response rate and geometric mean concentrations of anti-gE antibody (ATP cohort for immunogenicity). Samples were collected at the indicated time points (for both subcutaneous (SC) versus intramuscular (IM) groups) and anti-gE antibody concentrations were determined by ELISA. Data are vaccine response rates (VRRs) and geometric mean concentrations (GMCs) and error bars indicate 95% confidence interval.