Figures & data
Table 1. Base case results for palivizumab treated infants compared to no palivizumab treated infants by risk for severe RSV disease.
Table 2. Variables used in the model along with associated ranges used for the sensitivity analyses.
Figure 1. Tornado diagram of the relative impact on the ICER of each of the variables included in the model for a) All CF infants and b) the high risk CF infants only. The x-axis represents the range of the ICER when the parameters are varied by the ranges shown in brackets. The vertical line indicates the base-case ICER for PMB immunoprophylaxis among a) All CF infants (C$652,560 per QALY gained) and b) the high risk CF infants (C$157,332 per QALY gained).
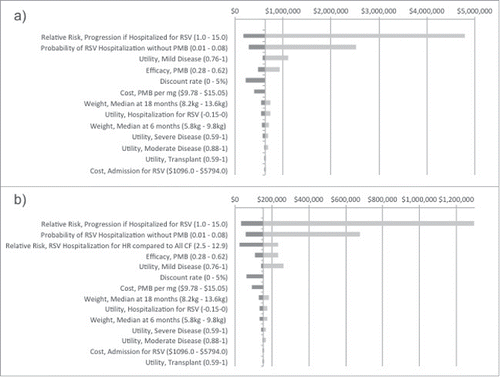
Figure 2. Two-way sensitivity analyses of they key parameters identified in one-way sensitivity analyses for the high-risk CF infants. Analyses were conducted using net benefit with a willingness to pay threshold of $50,000 per QALY. These graphs show the change in the optimal decision strategy over values of 2 variables: A) probability of RSV hospitalization vs. relative risk of progression if hospitalized with RSV, B) probability of RSV hospitalization versus effectiveness of PMB, C) cost of PMB per mg of body weight vs. PMB effectiveness, and D) cost of PMB per mg body weight versus probability of RSV hospitalization. The light gray region represents combinations of the variables for which the “No PMB” strategy is preferred while the dark gray region represents combinations of the variables for which the “PMB” strategy is preferred. The dotted lines represent the base-case values used in the analyses.
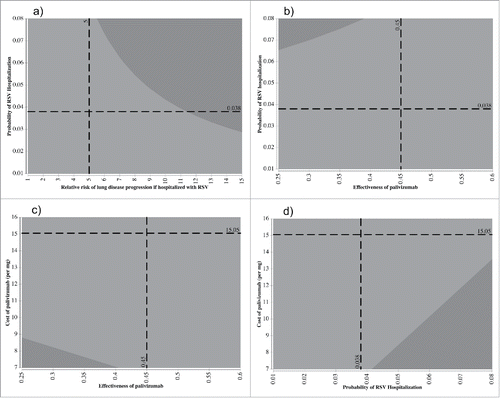
Figure 3. Model schematic for both the high risk and All CF groups. (HS1 = Mild Disease (FEV1 ≥ 70%), HS2 = Moderate Disease (40% ≤ FEV1 <70%), HS3 = Severe Disease 3 (FEV1 < 40%), HS4 = Heart and/or Lung Transplant).
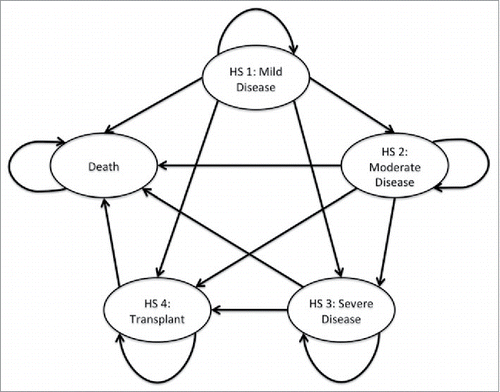
Figure 4. Comparison between model output (dashed line) and CF Canada statistics Citation41 (solid line) on cumulative probability of death for each age group.
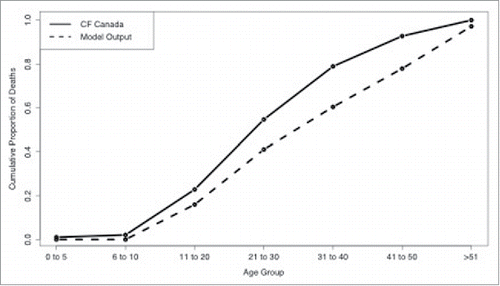
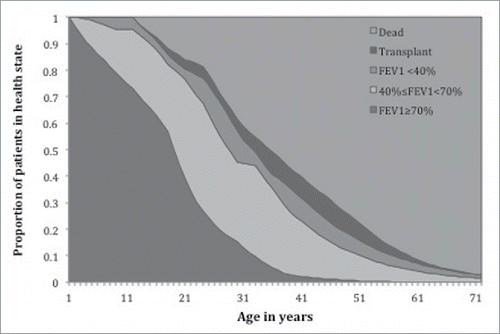
Figure 5. Health state distributions over the lifetime of the natural history cohort of All CF patients in the model. (HS1= Mild Disease (FEV1 ≥ 70%), HS2 = Moderate Disease (40%≤FEV1 < 70%), HS3 = Severe Disease 3 (FEV1 < 40%), HS4 = Heart and/or Lung Transplant).