Figures & data
Table 1. Summary of demographic characteristics (Total vaccinated cohorts).
Figure 1. Participant flow in the Primary and Booster vaccination studies. Group A, infants who received DTPa-IPV/Hib vaccine at 2, 3, 4 months of age; Group B, infants who received DTPa-IPV/Hib vaccine at 3, 4, 5 months of age; Control, infants who received DTPa/Hib and IPV vaccines at 2, 3, 4 months of age N, number of participants; TVC, total vaccinated cohort; ATP, according-to-protocol; SAE, serious adverse event; AE, adverse event. One participant was not included in the TVC due to consent withdrawal before vaccination.
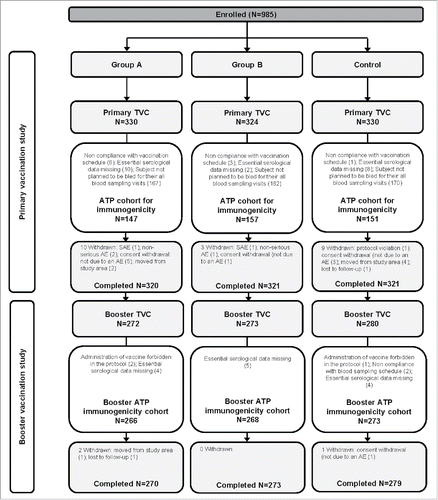
Figure 2. Solicited local and general symptoms reported within 4 d (day 0–3) after vaccination with DTPa-IPV/Hib in the Pilot study (A), or with DTPa-IPV/Hib or DTPa/Hib and IPV in the Primary vaccination study (B) or with or DTPa/Hib and IPV in the Booster vaccination study (C) (Total vaccinated cohorts). Group A, infants who received DTPa-IPV/Hib vaccine at 2, 3, 4 months of age; Group B, infants who received DTPa-IPV/Hib vaccine at 3, 4, 5 months of age; Control, infants who received DTPa/Hib and IPV vaccines at 2, 3, 4 months of age; %, percentage of participants. The error bars indicate 95% confidence intervals.
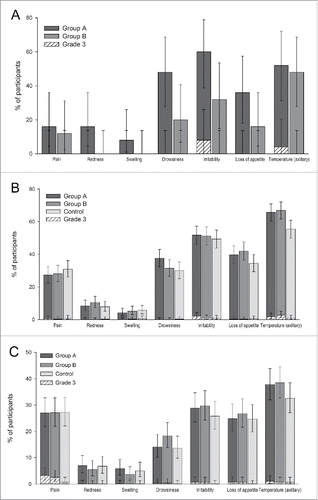
Table 2. Seroprotection/seropositivity rates and geometric mean antibody concentrations/titers before and one month after third dose/booster with DTPa-IPV/Hib or DTPa/Hib and IPV vaccines in the Primary and Booster vaccination studies (ATP cohort for immunogenicity).
