Figures & data
Figure 1. Long-term serum antibody response in mice after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21. Serum samples were collected before each immunization and at indicated time-points post-boost. HAI titers (A), MN titers (B), anti-HA IgG endpoint titers (C) and antibody avidity indices (D) against homologous virus was determined from individual mice. Differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent geometric means and 95% confidence intervals. (A) and (C) depict 8–16 mice per group. (B) and (D) depict 5–8 and 8–10 mice per groups, respectively.
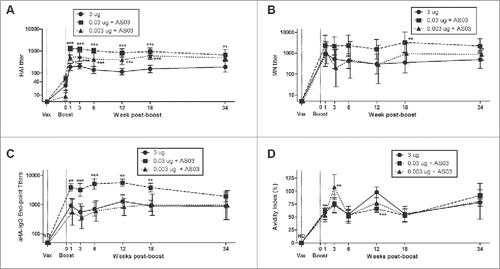
Figure 2. Generation and long-term maintenance of influenza HA-specific antibody secreting cells (ASCs) after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21. Splenocytes and bone marrow (BM) cells were isolated from individual mice at indicated time-points post-boost to investigate HA-specific IgG antibody secreting cells (ASCs) by ELISpot. The number (A) and spot size (B) of ASCs in splenocytes. The number (C) and spot size (D) of ASCs in BM cells. Representative ELISpot wells of cells isolated at12 weeks post-boost are shown in (E) and (F). Differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent means and standard errors, and depict 5–8 mice per group.
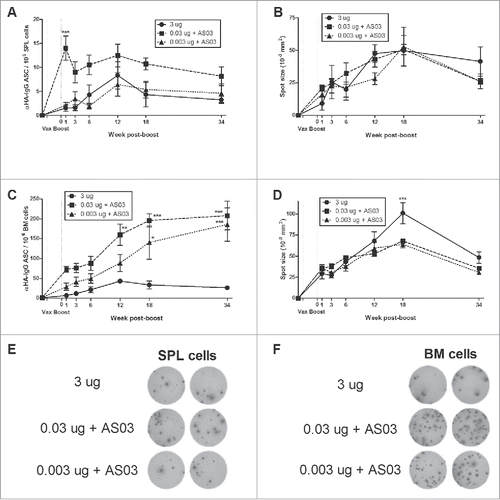
Figure 3. Generation and long-term maintenance of influenza HA-specific memory B cell-derived antibody secreting cells (ASCs) after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21. Splenocytes and bone marrow (BM) cells were isolated from individual mice at indicated time-points post-boost. Cells were stimulated ex vivo with CpG and human IL-2 to quantify the number and spot size of HA-specific memory B cell-derived ASCs (memory ASCs) by ELISpot. The number (A) and spot size (B) of memory ASCs in splenocytes. The number (C) and spot size (D) of memory ASCs in BM cells. Representative ELISpot wells of cells isolated at 34 weeks post-boost are shown in (E) and (F). Differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent means and standard errors, and depict 5–8 mice per group.
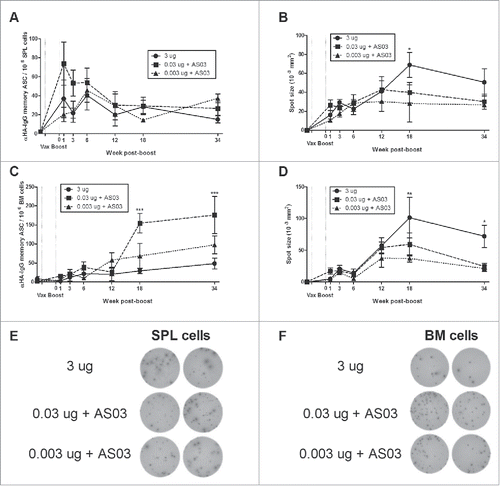
Figure 4. Unadjuvanted A/Uruguay H3N2 split vaccine and AS03-adjuvanted dose-sparing vaccines generate different antigen-specific cytokine/chemokine profiles over time. BALB/c mice were immunized intramuscularly on days 0 and 21, and splenocytes were isolated from individual mice at indicated time-points post-boost. Cells were stimulated ex vivo with media (unstimulated background) or with A/Uruguay H3N2 split vaccine and culture supernatant was collected after 72 hours. The concentrations of indicated cytokines/chemokines in supernatants were determined using Q-Plex™ Mouse Cytokine - Screen (16-plex) multiplex ELISA. Cytokine/chemokine profiles at 1, 3, 18 and 34 weeks post-boost are shown. For each cytokine/chemokine, concentrations are shown as the fold increase from “Unstim,” which represents the average concentration of unstimulated samples for all groups at all time-points. The concentration of “Unstim” background is indicated in brackets next to cytokine/chemokine names. Individual cytokine/chemokine concentrations at all time-points are shown in Fig S3. Data represent means and standard errors, and depict 5–8 mice per group.
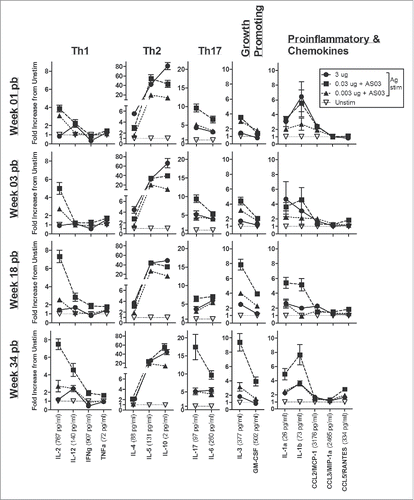
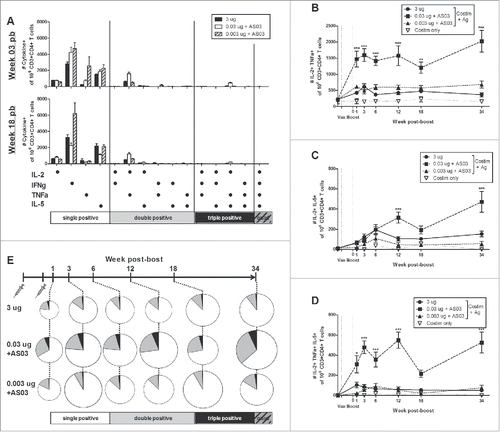
Figure 5. Long-term generation of antigen-specific CD4+ T cells after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21, and splenocytes were isolated from individual mice at indicated time-points post-boost. Cells were stimulated ex vivo with A/Uruguay H3N2 split vaccine and co-stimulatory antibodies, then analyzed by flow cytometry for CD4+ T cells that produced a combination of IL-2, IFNγ, TNFα, or IL-5 cytokines. The gating strategy is described in Fig. S4. Frequency of CD4+ T cells expressing all combinations of IL-2, IFNγ, TNFα, or IL-5 cytokines at week 3 and 18 post-boost (A). The frequency of IL-2+TNFa+ double positive (B), IL-2+IL-5+ double positive (C) or IL-2+TNFa+IL-5+ triple positive (D) CD4+ T cells. The distribution of CD4+ T cell subsets based on the number of cytokines expressed (E). The size of the pie chart is also representative of the magnitude of the total response. For (B), (C), and (D), differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent mean and standard errors, and depicts 5–8 mice per group.