Figures & data
Table 1. Ten-year average annual results for the base-case analysis (2012–2022): IIV4 versus IIV3.
Table 2. Ten-year average annual scenario analyses results (2012–2022)Footnotea.
Figure 1. Simplified model structure for cost-effectiveness of IIV4 versus IIV3 (the full structure can be found in Thommes et al.Citation22). *A proportion of individuals were vaccinated with either IIV3 or IIV4 according to the vaccine coverage parameters shown in . †Case of influenza type A or B. ‡ED cases were set to zero due to a lack of robust data. [+] indicates clinical pathway is the same as above. ED, emergency department; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; LRTC, lower respiratory tract complication; OM, otitis media.
![Figure 1. Simplified model structure for cost-effectiveness of IIV4 versus IIV3 (the full structure can be found in Thommes et al.Citation22). *A proportion of individuals were vaccinated with either IIV3 or IIV4 according to the vaccine coverage parameters shown in Figure. 2. †Case of influenza type A or B. ‡ED cases were set to zero due to a lack of robust data. [+] indicates clinical pathway is the same as above. ED, emergency department; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; LRTC, lower respiratory tract complication; OM, otitis media.](/cms/asset/b1ad0e01-4fef-4e1d-8fae-18f5cba40981/khvi_a_1242541_f0001_b.gif)
Figure 2. HistoricalCitation10-14 and projected influenza vaccine coverage by age group. Historical vaccine coverage data were available through 2012, and data were not available for every year for children aged <18 years; therefore, vaccination coverage estimates for future years and for years without historical estimates were projected using regression techniques and available historical data. Exponential and logarithmic functions were tested, and best-fit functions were selected for each age group.
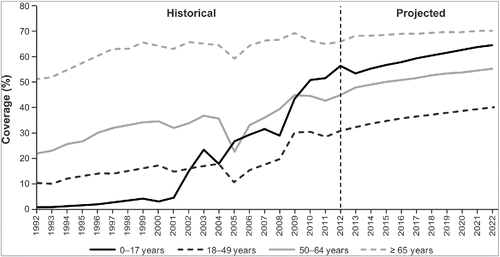
Table 3. Vaccine-related inputs.
Table 4. Influenza-related inputs (for type A and type B influenza).
