Figures & data
Figure 1. Flow diagram of subject enrollment and completion. Subjects were ineligible to receive second injection if they experienced a grade 3 adverse event or reactogenicity event after the first injection (all events determined probably unrelated to the first injection). For subjects in the 50 µg bivalent cohort (active vaccine, saline placebo, or alum placebo recipients) who received a second injection, the follow-up phone call and study completion occurred on Day 252.
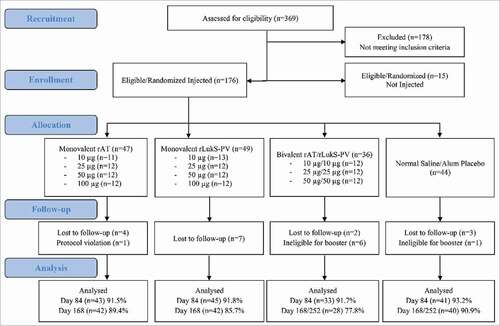
Figure 2. Local or systemic reactogenicity (A) incidence and (B) severity during the first week following first vaccination in all treated subjects (n = 176).
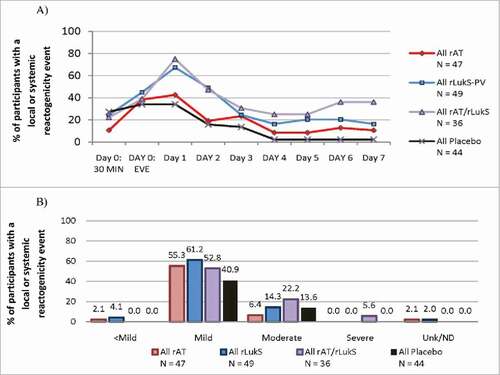
Table 1. Summary of adverse events following first vaccination during follow-up in the safety population, No. (%). There was no significant difference in the incidence of overall AEs or AE's assessed to be treatment-related among the four main treatment groups, except subjects with ≥ 1 severe AE (p<0.01). p-values were calculated using a Wald test statistic for group in a generalized linear model for count outcomes
Figure 3. Antibody responses for (A) monovalent rAT; (B) bivalent rAT; (C) monovalent rLukS-PV; and (D) bivalent rLukS-PV formulations following first and second vaccination in the completer population. Visits are based on pre-defined windows for serology sample collection and not on the nominal visit. All out-of-window sample collections are excluded). After Day 84, the number of subjects who received the booster dose in the bivalent 50 µg cohorts was six.
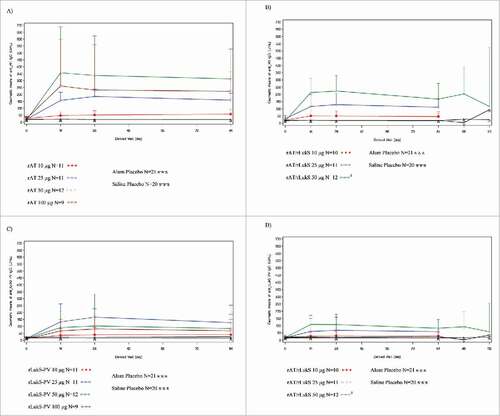
Figure 4. a) Geometric mean titer of rAT neutralizing antibody b) Geometric mean titer of rLukS-PV neutralizing antibody following vaccination. Volunteers were vaccinated with 25 µg rLukS-PV, 50 µg rLukS-PV, 25µg rAT/rLukS-PV or 50 µg rAT/rLukS-PV. The 50 µg rAT/rLukS-PV cohort received a booster dose on day 84, and evaluation of neutralizing antibodies at day 112.
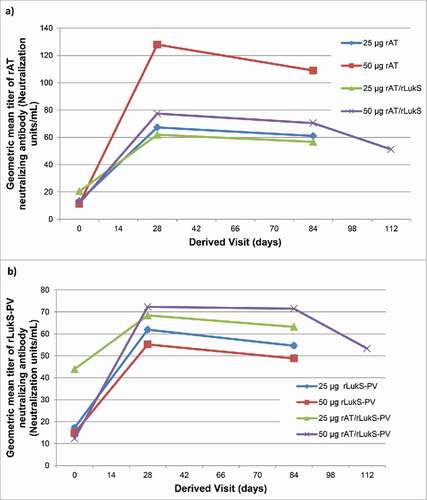
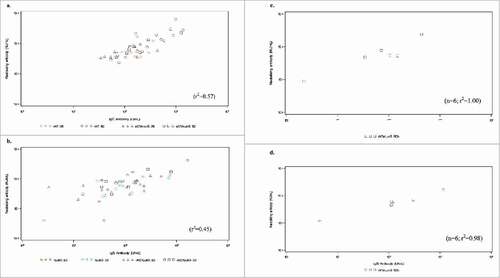
Figure 5. Scatterplot of total IgG levels and neutralizing activity at Day 84 post-vaccination against rAT for the following doses: 25 µg rAT, 50 µg rAT, 25 µg rAT/rLukS-PV or 50 µg rAT/rLukS-PV cohorts (5a), rLukS-PV for the 25 µg rLukS-PV, 50 rLukS-PV, 25 µg rAT/rLukS-PV or 50 µg rAT/rLukS-PV cohorts (5b). Scatterplot of total IgG levels and neutralizing activity against rAT (5c) and rLukS-PV (5d) at Day 112 following receipt of a booster dose of vaccine in patients who received the 50 µg rAT/rLukS-PV dose at baseline.