Figures & data
Table 1. Characteristics of study participants (Total vaccinated cohort).
Figure 2. Vaccine response rates for anti-gE antibody concentrations one month after the second vaccine dose: overall, by age group and by time since previous herpes zoster episode (ATP cohort for immunogenicity).
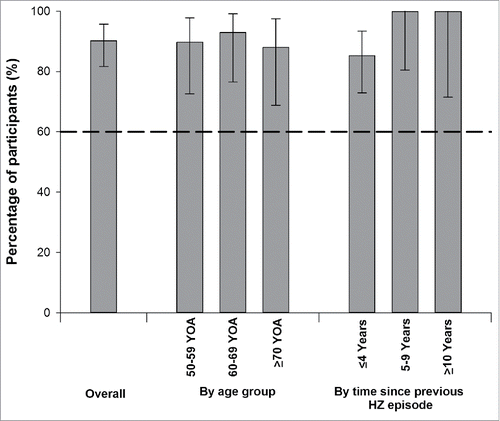
Table 2. Vaccine response rates and geometric mean concentrations of anti-gE antibodies at Month 0 and Month 3 (ATP cohort for immunogenicity).
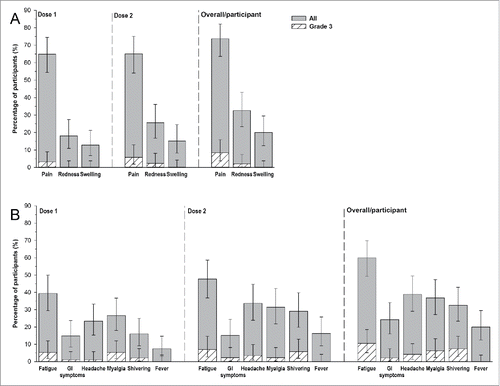
Figure 3. Incidence of solicited local (A) and general (B) adverse events (total vaccinated cohort, overall/participant).
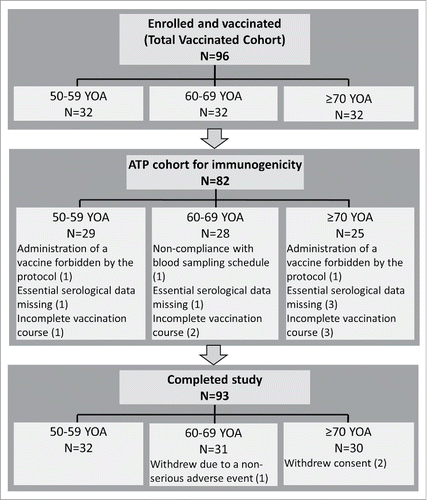
Table 3. Overview of suspected HZ episodes reported during the study (total vaccinated cohort).