Figures & data
Figure 1. Immunogenicity and protective efficacy of CP5 and CP8 conjugate vaccines in BALB/c mice. Groups of four BALB/c mice were immunized on days 0, 14, and 28 with 10, 2, or 0.4 µg of CP5-CRM, CP8-CRM, or CRM alone adsorbed to 100 µg aluminum phosphate adjuvant. Blood was collected from each mouse before each immunization and prior to challenge. Serum was diluted 1:100 for evaluation of antibodies to CP5 or CP8 by ELISA (A). On Day 42, the mice were challenged intraperitoneally with ∼107 CFU of (C) Reynolds (CP5) or (D) Reynolds (CP8). Quantitative blood cultures were performed on heparinized blood collected by tail vein puncture 2 h after challenge. Data are presented as means ± standard errors and were analyzed by one-way ANOVA with multiple comparisons made to the group receiving CRM alone. ****, P < 0.0001; *, P < 0.05. For each experiment, the results of one representative experiment are presented; each was repeated at least twice.
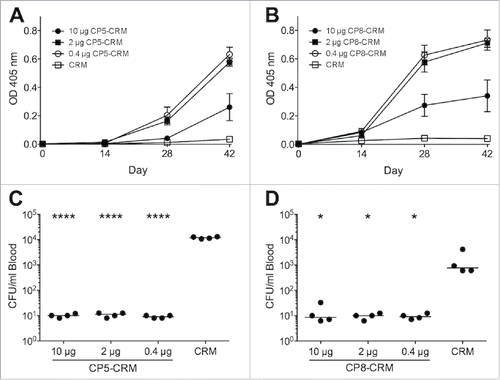
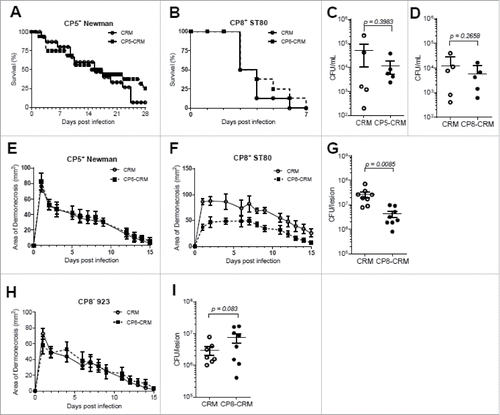
Figure 2. Evaluation of CP5-CRM and CP8-CRM as vaccines against S. aureus strains Newman and ST80 in the murine models of lethal sepsis and skin infection. BALB/c mice were immunized with 1 µg of CP5-CRM or CP8-CRM adsorbed to 100 µg aluminum phosphate adjuvant on days 0, 14, and 28. On day 42, mice were challenged in the bacteremia model with (A) Newman or (B) ST80 and in the dermonecrosis model with (E) Newman or (F) ST80 (n = 8 to 10 mice/group). In the lethal sepsis model, blood was collected at 24 hours post-infection and plated for CFU analysis (C-D) and mice were monitored for survival for 28 days. In the skin infection model, the area of dermonecrosis was photographed and measured for 15 days. (G) Bacterial burden on 7 days post-infection in the lesions of CP8-CRM or CRM immunized BALB/c mice challenged with ST80 in the skin infection model. (H) Immunized mice were challenged in the skin infection model with USA300 strain 923 (n = 8/group). (I) Bacterial burden on 7 days post-infection in the skin lesions of CP8-CRM or CRM immunized BALB/c mice challenged with USA300 strain 923. Data are presented as means ± standard errors and considered significant at a confidence level of 95%. For each experiment, the results of one representative experiment are presented; each was repeated at least twice.