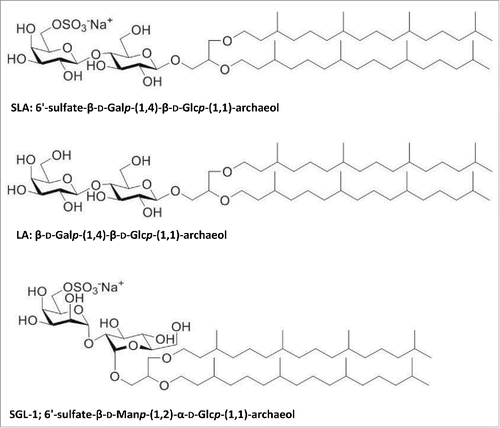
Figures & data
Figure 2. Comparison of CD8+ (T)cell responses induced by OVA entrapped in SLA and SGL-1 archaeosomes. C57BL/6 mice (n = 6/gp) were immunized with 20 µg OVA entrapped in SLA or SGL-1 archaeosomes on days 0 and 21. At 3 weeks post second immunization, representative mice (n = 2 per group) were killed, splenocytes isolated, stimulated with IL-2 (0.1 ng/mL) and OVA257–264 (10 μg/ml) and the frequency of IFN-gamma secreting cells in triplicate cultures enumerated by ELISPOT. Number of spots/106 spleen cells is indicated.
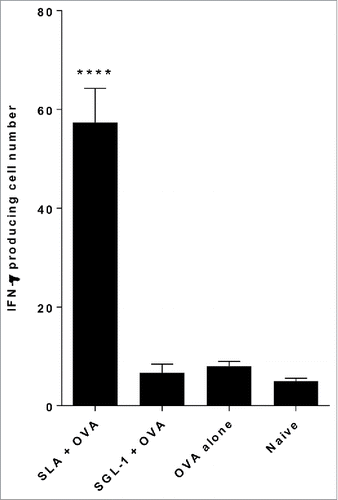
Figure 3. Immune responses induced by OVA entrapped in different archaeosomes. C57/BL6 mice (n = 6/gp) were immunized with 20 µg OVA alone or entrapped in SLA, SLA:LA or M. smithii archaeosomes on days 0 and 21. At 3 weeks post second immunization, serum was collected and OVA-specific IgG titers measured by ELISA (panel A). Representative mice (n = 2 per group) were killed and splenocytes isolated. Pooled splenocytes were stimulated with IL-2 (0.1 ng/mL) and OVA257–264 (10 μg/ml) and the frequency of IFN-gamma secreting cells in triplicate cultures enumerated by ELISPOT. Number of spots/106 spleen cells is indicated (panel B). Splenocytes were stimulated with OVA275–264 for 5 d before assessing CTL activity against 51Cr-labeled targets. CTL data represent percentage of specific lysis of triplicate cultures ± SD at various E:T ratios (panel C).
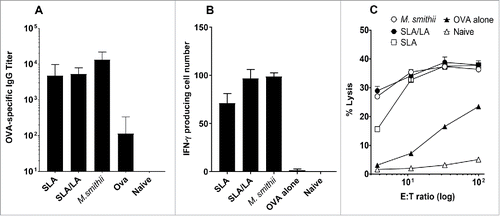
Figure 4. Immune responses induced by OVA entrapped in different SLA/LA archaeosomes. C57/BL6 mice (n = 6/gp) were immunized with 20 µg OVA entrapped in SLA, SLA:LA (at various ratios) or M. smithii archaeosomes on days 0 and 21. At 3 weeks post second immunization, serum was collected and OVA-specific IgG titers measured by ELISA (panel A). Representative mice (n = 2 per group) were killed and splenocytes isolated. Pooled splenocytes were stimulated with IL-2 (0.1 ng/mL) and OVA257–264 (10 μg/ml) and the frequency of IFN-gamma secreting cells in triplicate cultures enumerated by ELISPOT. Number of spots/106 spleen cells is indicated (panel B). Splenocytes were also stimulated with OVA275–264 for 5 d before assessing CTL activity against 51Cr-labeled targets. CTL data represent percentage of specific lysis of triplicate cultures ± SD at various E:T ratios (panel C). Zeta potential (mV) was measured as an indicator of archaeosomes stability and plotted relative to %SLA in different archaeosomes (panel D). The zeta potential (mV) of each archaeosome was also plotted against its respective CD8+ T cell response, as measured by the frequency of IFN-gamma secreting cells enumerated by ELISPOT (panel E).
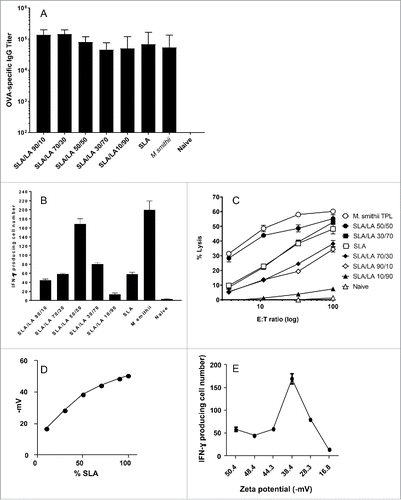
Table 1. Archaeosome vaccine characteristics and stability.
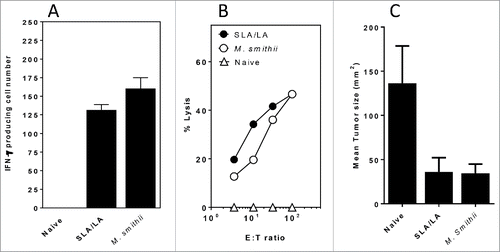
Figure 5. CD8+ (T)cell responses and protection against B16 melanoma challenge induced by TRP-2 entrapped in different archaeosomes. C57/BL6 mice (n = 6/gp) were immunized 15 µg tyrosinase-related protein 2 entrapped in SLA:LA or M. smithii archaeosomes on days 0, 25 and 60. At ∼11 weeks post first immunization, representative mice (n = 2 per group) were killed and splenocytes isolated. Pooled splenocytes were stimulated with IL-2 (0.1 ng/mL) and TRP-2180–188 (10 μg/ml) and the frequency of IFN-gamma secreting cells in triplicate cultures enumerated by ELISPOT. Number of spots/106 spleen cells is indicated (panel A). Splenocytes were also stimulated with TRP2180–189 for 5 d before assessing CTL activity against 51Cr-labeled targets. CTL data represent percentage of specific lysis of triplicate cultures ± SD at various E:T ratios (panel B). Remaining mice (n = 4/gp) were injected with B16 melanoma tumor cells and tumor size monitored over time. The mean tumor size at day 14 following tumor cell injection is shown (panel C).