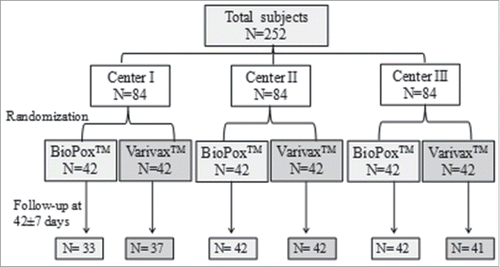
Figures & data
Table 1. Seroconversion: Comparative analysis and non inferiority analysis for subjects with pre-immune titer <10 mIU/mL cohort I and II receiving Bio Pox™ and Varivax™ at all study centers.
Table 2. Adverse events (local, systemic and skin reactions) and post-immunization safety profile recorded for combined cohort I (> 6 to 12 years) and cohort II (1 to 6 years) at different time intervals of post vaccination for Bio Pox™ and Varivax™ at all study centers.