Figures & data
Figure 1. Specificity and sensitivity of allergen-specific antibodies. A, Reactivity of the antibodies with their corresponding recombinant target allergens in ELISA. B, Reactivity of the antibodies with their native target allergens in crude honeybee venom. In comparison, the IgE-reactivity of a poolserum from honeybee venom-allergic patients is shown. Since all investigated allergens represent glycoproteins, the molecular weights do not correspond to that of the calculated weights of the protein portions only, which are stated in some databases. C, Detection of the particular allergens in serial dilutions of crude honeybee venom to assess the sensitivity of the allergen-specific antibodies in immunoblot.
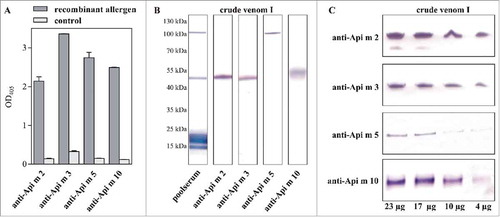
Figure 2. Allergen content of therapeutic honeybee venom extracts. A, Allergen content of therapeutic venom extracts compared with crude venom as assessed by the use of polyclonal (Api m 2, Api m 3 and Api m 10) and monoclonal (Api m 5) antibodies. Representative results of 5 batches of Venomil (Allergy Therapeutics, Worthing, UK), 2 batches Reless (Pharmalgen) (ALK-Abelló, Hamburg, Germany), 2 batches ALK lyophylisiert SQ (Aquagen SQ) (ALK-Abelló) and 2 batches of Venomenhal (HAL Allergy, Leiden, Netherlands) are shown. Ponceau S staining of Api m 1 served as loading control (a representative staining is shown). B, Api m 3 and Api m 10 content of 3 independent batches (B1-B3) of Venomenhal.
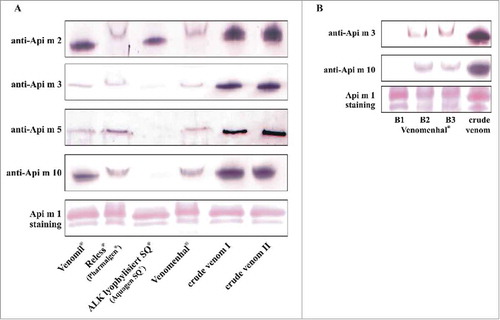
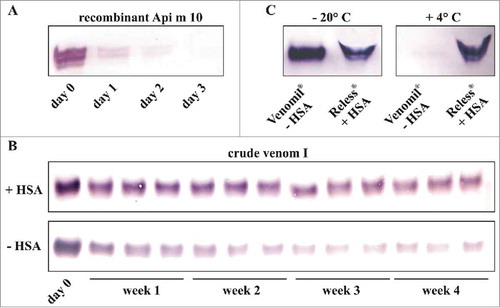
Figure 3. Stability of the major allergen Api m 10. A, Stability of recombinant purified Api m 10 produced in insect cells. Api m 10, in a concentration comparable to that detected in crude venom, was stored for 3 d at + 4° C. B, Stability of Api m 10 in crude honeybee venom reconstituted either with HSA-containing diluent for injection (Allergy Therapeutics) or PBS upon storage at + 4° C for 4 weeks. C, Detection of Api m 10 in water-resolved Venomil (no HSA in the lyophylisate) and Reless (HSA in the lyophylisate) stored for 4 weeks at either - 20° C or + 4° C.