Figures & data
Figure 1. Development of the recombinant Hgp44 antigen. (A) The purity of Hgpp44 recombinant antigen was confirmed by SDS-PAGE. (B) Western blot analysis of the purified antigen using pre-immune and anti-P. gingivalis serum. (C) Evaluation of the Hgp44 polypeptide expression in P. gingivalis (ATCC33277) lysate was performed by western blotting using anti-Hgp44 while Fusobacterium nucleatum (ATCC 10953) lysate was used as control. (D) The hemagglutination inhibition assay (HIA). P. gingivalis was pre-incubated with the diluted Hgp44 antisera and then mixed with an equal volume of mouse RBC solution. The anti-Pg sera were used as a hemagglutination inhibitor control.
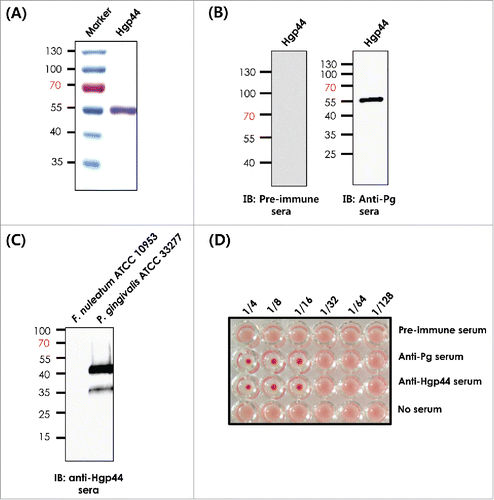
Figure 2. Determination of the Hgp44-specific antibody titers by ELISA. The anti-Hgp44-specific serum IgG (A) and saliva IgA (B). The data are presented as the mean ± SEM in each group. N = 5. *P < 0.05, **P < 0.01, ***P < 0.001, NS: non-significant, ND: not detected (under the detection limit).
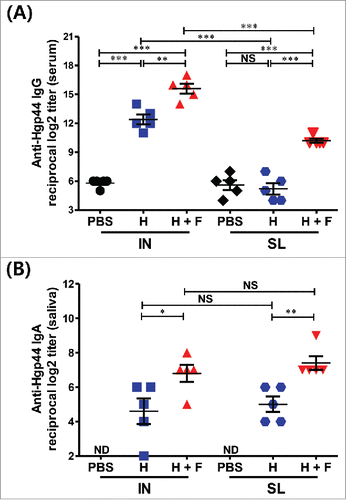
Figure 3. Protective immune response induced by flagellin-adjuvanted Hgp44 mucosal vaccination in a mouse P. gingivalis infection model. The mice were intranasally or sublingually immunized as described above. One week after the final immunization, the immunized mice were orally challenged with P. gingivalis (ATCC 33277) for 3 successive days as described in the Materials and Methods section. Forty days after the final challenge, the alveolar bone loss was determined by micro-CT. (A) Analysis of the micro-CT volumetric parameter (bone volume/tissue volume; BV/TV) (n = 8). The ROI was defined as a cuboidal bone body encompassing the roots of the M1 and M2 isolated from intranasally vaccinated or sublingually vaccinated mice. n = 4–6. (B) Determination of the distance from the CEJ to the ABC in the maxillae. Forty days after the final challenge, the alveolar bone loss was determined using micro-CT by measuring the distance from the CEJ to the ABC with M2. The data are presented as the mean ± SEM in each group. Naïve n = 4; PBS (IN) n = 14; H (IN) n = 7; H + F (IN) n = 8; PBS (SL) n = 16, H (SL) n = 8; H + F (SL) n = 7) *P < 0.05, **P < 0.01.
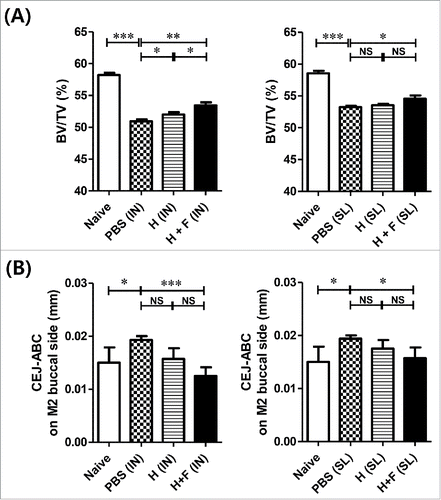
Figure 4. Development and evaluation of the recombinant fusion antigens. (A) SDS-PAGE of the recombinant fusion proteins. (B) Determination of the TLR5-dependent NF-κB stimulating activity by fusion proteins. Fusion-protein-mediated TLR5 signaling was determined using an NF-κB-luciferase reporter assay. The 293 cells were co-transfected with hTLR5 and pNF-κB-Luc and further treated with FlaB, FlaB-Hgp44 (FH) or Hgp44-FlaB (HF) for 24 h. The relative luciferase activities in the cell extracts were analyzed by a dual-luciferase reporter assay system and normalized using the pCMV-β-galactosidase plasmid as a control. The data are presented as the mean ± SEM in each group. ** P < 0.01, *** P < 0.001.
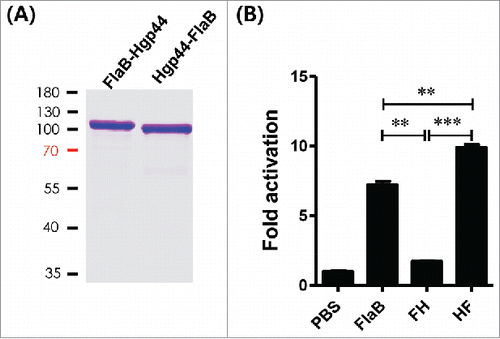
Figure 5. The Hgp44-specific antibody titers induces by IN vaccination. The mice were intranasally immunized with PBS, 4 μg of Hgp44 alone (H) or 4 μg of FlaB along with 4 μg of Hgp44 (H+F), and 8 \mu{g} Hgp44-FlaB fusion protein (HF); 3 times at 2-week intervals. Two weeks after the final vaccination, an ELISA was performed to determine the Hgp44-specific antibody. Determination of the anti-Hgp44-specific serum IgG (A) and saliva IgA (B) titers. The data are presented as the mean ± SEM in each group. n = 5. ** P < 0.01, *** P < 0.001, (NS) non-significant.
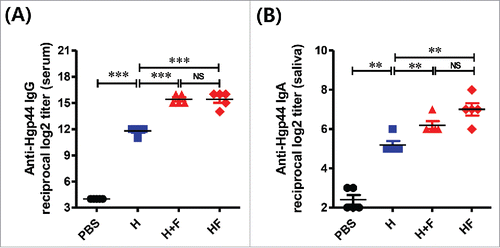
Table 1. Bacterial strains and plasmids used in this study.
