Figures & data
Figure 1. Study Design for the CryJ2-LAMP trial. Route of Administration – intramuscular (IM); * Average of days from the visit #2 to the #8; ** Average 166 d from the visit #8 to the visit #9.
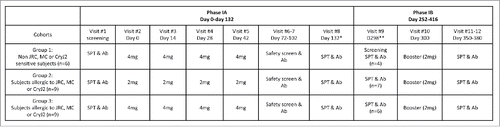
Table 1. Demographics and Baseline Characteristics.
Table 2. Summary of all TEAEs by Cohort (day 0 - day 132).
Table 3. Summary of all AEs during the Phase IB Trial.
Table 4. Skin Prick Test Results for JRC and MC.
Table 5. Skin Prick Test Results for Allergens Unrelated to JRC and MC.
