Figures & data
Figure 1. Relevant species and allergens in hymenoptera venom allergy. A, Taxonomy of hymenoptera, Citation154,Citation155 with examples of prominent species, which are relevant elicitors of venom allergy. B, Identified allergens of the allergy-relevant hymenoptera species Polistes dominula, Vespula vulgaris and Apis mellifera. Allergens which are marked with an asterisk are available for routine diagnosis. Indicated in red are commercially available marker allergens, used to discriminate between allergies against Polistes/Vespula and honeybee venom allergy. Cross-reactive allergens and their sequence identity (in percent) are shown in gray boxes.
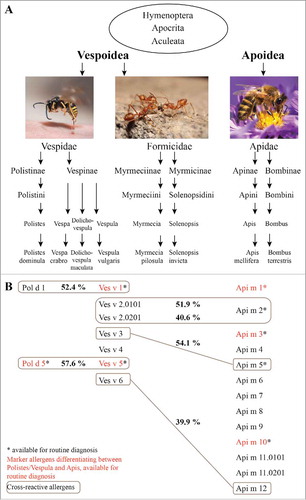
Table 1. Identified venom allergens from honeybee (Apis mellifera), yellow jacket (Vespula vulgaris) and paper wasp (Polistes dominula). MW, molecular weight; CRP, carbohydrate-rich protein; DPP IV, Dipeptidyl peptidase IV; DW, dry weight; MRJP, major royal jelly protein.
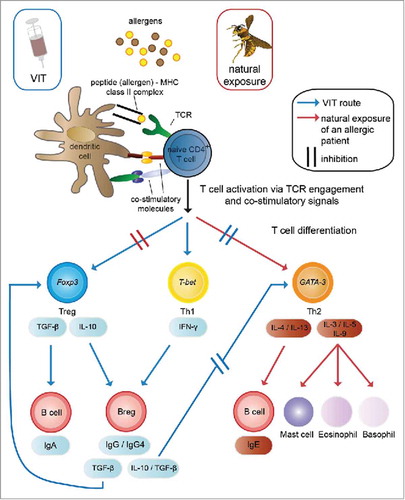
Figure 2. Mechanisms of venom-specific immunotherapy (VIT) compared with the allergic immune response. Venom allergens are injected into the skin, either by the allergy-causing insect (natural exposure) or by subcutaneous injection during VIT. Skin-resident dendritic cells take up the allergens, process them and present derived peptides in a complex with MHC class II molecules to allergen-specific naive CD4+ T cells. In a venom-allergic individual this leads to their differentiation into Th2 cells (key transcription factor: GATA-3), which secrete the cytokines IL-3, IL-5 and IL-9, contributing to the activation and degranulation of mast cells, eosinophils and basophils, as well as IL-4 and IL-13 which induce the production of IgE by B cells. These inflammatory processes elicit the allergic reaction and suppress a tolerogenic phenotype of the immune response, which can be observed in individuals not allergic to insect venom. A shift of this Th2-directed reaction toward a tolerogenic reaction is observed during VIT, characterized by the differentiation of allergen-specific naive T cells to Tregs (key transcription factor: Foxp3) and Th1 cells (key transcription factor: T-bet). The effector cytokines of Tregs and Th1 cells then lead to the suppression of Th2 cells and their inflammation-promoting functions, therefore causing desensitization of mast cells and basophils, as well as the induction of IgA-producing B cells and Bregs. These Bregs then produce protective blocking IgG4 antibodies, further enhance the differentiation of Tregs via TGF-β and can inhibit the differentiation of Th2 cells via IL-10 and TGF-β. Citation99,Citation100,Citation102-Citation104 Breg: regulatory B cell; Foxp3: Forkhead Box P3; GATA-3: GATA binding protein 3; Ig: Immunoglobulin; IL: interleukin; MHC: major histocompatibility complex; T-bet: T-Box 21; TCR: T cell receptor; TGF-β: Transforming growth factor β; Th: T helper cell; Treg: regulatory T cell; VIT: venom-specific immunotherapy.