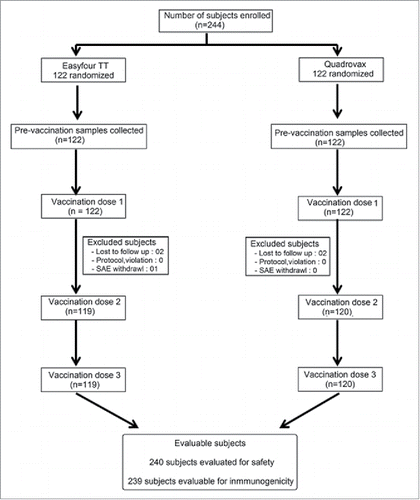
Figures & data
Table 1. Demographic profile of subjects.
Table 2. Seroprotection/ Seroconversion rates (SPR) 4 weeks post-vaccination.
Table 3. Geometric mean titer (GMT) and 95% CI.
Table 4. Total incidences of solicited local and systemic AEs reported during the study period (number and percentage of subjects experiencing at least one AE).