Figures & data
Figure 1. Participant disposition and study flow in the randomized cohort (adults ≥ 65 y of age). A total of 675 adults ≥ 65 y of age were randomly assigned 1:1:1 to receive the quadrivalent split-virion influenza vaccine (IIV4), the licensed trivalent split-virion influenza vaccine containing the B Victoria-lineage strain (IIV3–1), or an investigational split-virion IIV3 containing the B Yamagata-lineage strain (IIV3–2). AE, adverse event; n, number of participants in the group.
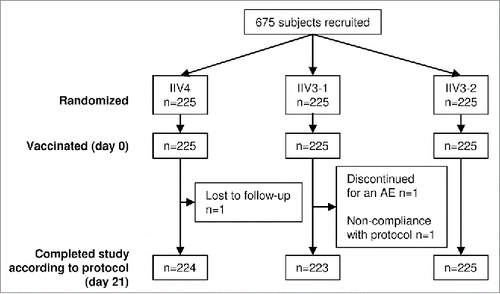
Table 1. Participant characteristics in the randomized cohort (≥ 65 years).
Table 2. Immunogenicity comparisons in the randomized cohort (≥ 65 years).
Table 3. Immunogenicity measures in the randomized cohort (≥ 65 years).
Table 4. Proportions reporting solicited reactions in the randomized cohort (≥ 65 years).
Table 5. Proportions reporting unsolicited adverse events in the randomized cohort (≥ 65 years).
