Figures & data
Figure 1. Survival curve, serum viremia, morbidity scores, and body weight and temperature changes for NHPs post-exposure. A) All of the DNA-vaccinated NHPs survived, while 2 of the 4 mock-vaccinated NHPs survived to the study end point. B) Overall morbidity scores assigned daily for each NHP as a subjective measure of observed responsiveness and presence or absence of disease signs; C) Serum viremia post-exposure as measured by plaque assay and expressed as the Log10 pfu/ml per blood sample collection day; D) Changes in body weight expressed as a percentage change from baseline weights for each NHP, then averaged per group; and E) Changes in body temperature as measured by rectal and/or temperature transponder chips and expressed as a percentage change from baseline temperatures for each NHP, then averaged per group.
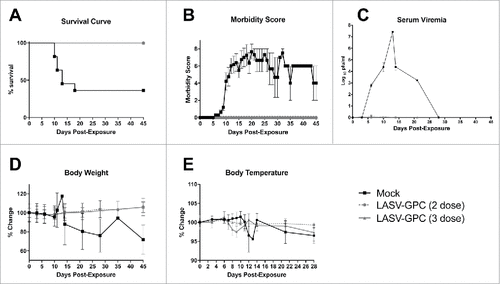
Figure 2. LASV-specific neutralization pre- and post-exposure. Neutralizing antibodies were quantified by PRNT50. Results are expressed as the reciprocal of the serum dilution resulting in 50% reduction in plaques compared with virus-only control wells.
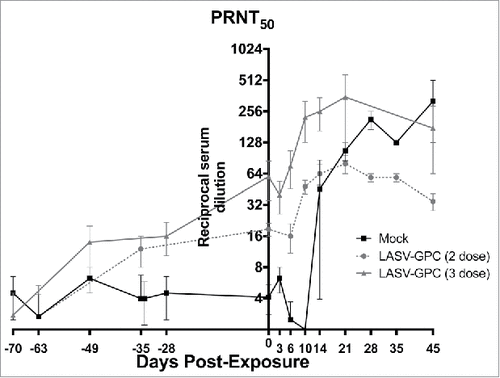
Figure 3. Changes in selected complete blood count counts after LASV exposure. A) White blood cells; B) Neutrophils; C) Lymphocytes; D) Monocytes; E) Eosinophils; F) Basophils; G) Hemoglobin; H) Hematocrit; I) Platelets. ![]()
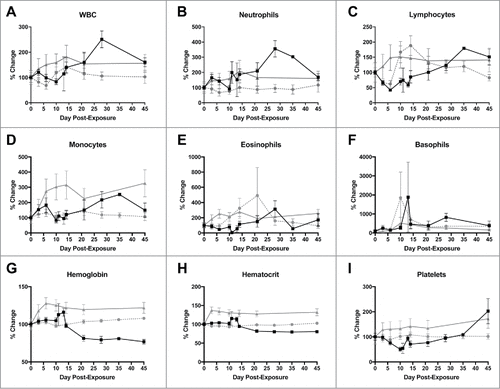
Figure 4. Changes in selected blood chemistry values after LASV exposure. A) Blood urea nitrogen; B) Creatinine; C) Total bilirubin; D) Albumin; E) Total protein; F) Gamma-glutamyltransferase; G) Alanine aminotransferase (ALT); H) Aspartate aminotransferase (AST); and Alkaline phosphatase (ALP). ![]()
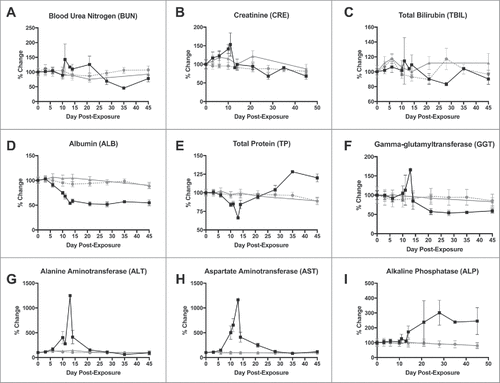
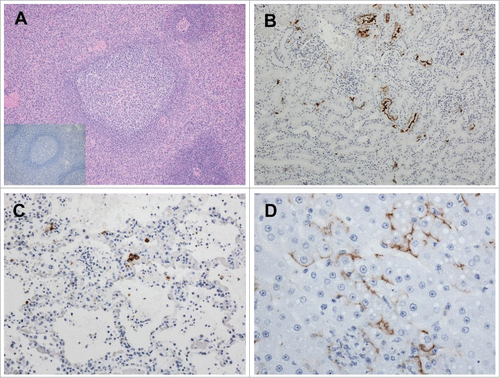
Figure 5. Pathologic analysis of selected tissues in LASV-exposed NHPs. A) Spleen, peri-arteriolar lymphoid sheath (PALS) hyperplasia in a vaccinated macaque that survived LASV challenge, 4X magnification; Inset, spleen, PALS hyperplasia with a complete absence of LASV immunoreactivity; B) Kidney, positive cytoplasmic immunoreactivity in islet and exocrine epithelial cells Of an NHP that succumbed on Day 11 post-exposure, 20x; C) Lung, cytoplasmic immunoreactivity in low numbers of alveolar macrophages, pneumocytes and endothelial cells in an NHP that succumbed on Day 17 post-exposure, 20x; D) Liver, positive apical to membranous hepatocyte and cytoplasmic endothelial immunoreactivity in an NHP that succumbed on Day 11 post-exposure, 40x.