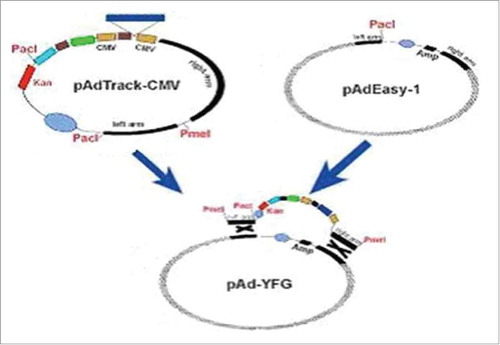
Figures & data
Figure 1. A. Electrophoresis results for GM-CSF and LMP2A. Lane 1 = DNA DL2000 Marker; lanes 2 and 3 = GM-CSF gene; lane 4 = DNA DL2000 Marker; lane 5 = LMP2A gene. B. Electrophoresis results for the fusion gene GC2A. Lane 1 = DNA DL2000 Marker; lanes 2 and 3 = GC2A genes. C. Identification of pAdTrack-CMV-GC2A. Lane 1 = enzyme analysis after Bg1II and EcoRV digest; lane 2 = DNA DL2000 Marker; lane 3 = pAdTrack-CMV-GC2A. D. Restriction enzyme digestion for pAd-GC2A analysis and identification. Lane 1 = DNA DL15000 Marker; lane 2 = PacI enzyme digestion of recombinant vector.
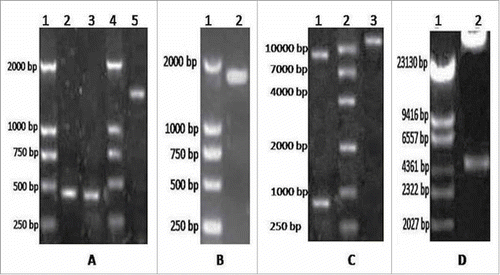
Figure 2. Delection of the fusion gene GM-CSF and LMP2A expression in recombinant adenovirus. A. The 293 cells transfected by recombinant adenovirus vectors (200 ×,56 h). B. Western blotting analysis of expressed (Lane 1 = western blotting with GM-CSF of Adenovirus 5 control; lane 2 = western blotting with GM-CSF of vAd-GC2A; lane 3 = Protein Markers; lane 4 = western blotting with LMP2A of Adenovirus 5 control; lane 5 = western blotting with LMP2A of vAd-GC2A; lane 6 = protein markers).
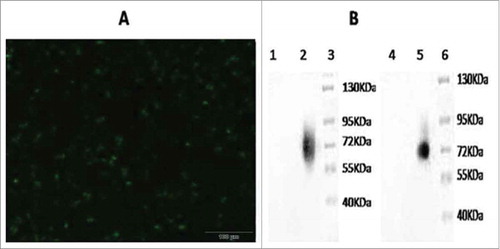
Figure 3. Specific IgG antibody response of mice immunized with recombinant adenovirus by intraperitoneal injection. Recombinant adenovirus maximum dose was 5 × 108 virus particles/100 μl, recombinant adenovirus secondary dose was 5 × 107 virus particles/100 μl, and PBS control dose was 100 μl. The recombinant adenovirus maximum dose exerted the most proliferative effect, antibody titers peaked on week 7.
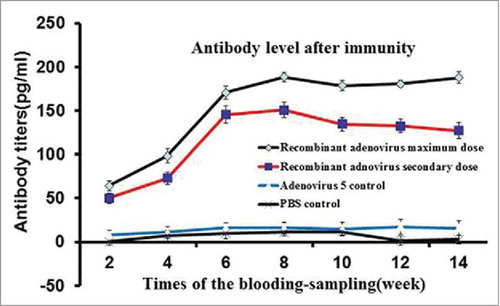
Table 1. Specific CTL killing activity of immunized mice (%).
Figure 4. Tumor size. The adenovirus vaccine group was injected with 2 × 108 plaque-forming unit recombinant adenovirus, other groups were injected with 2 × 108 plaque-forming unit (adenovirus 5+ LMP2A), (adenovirus 5+ hGM-CSF), (adenovirus 5+ plasmid) (soluble in 250 μL PBS)/mouse, respectively. The adenovirus 5 control group was injected with 2 × 108 plaque-forming unit adenovirus 5 (soluble in 250 μL PBS)/mouse, the PBS control group was injected with 250 μL PBS/mouse.
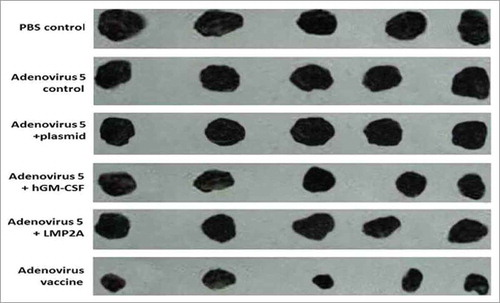
Table 2. Subcutaneous tumor model results (P < 0.01).
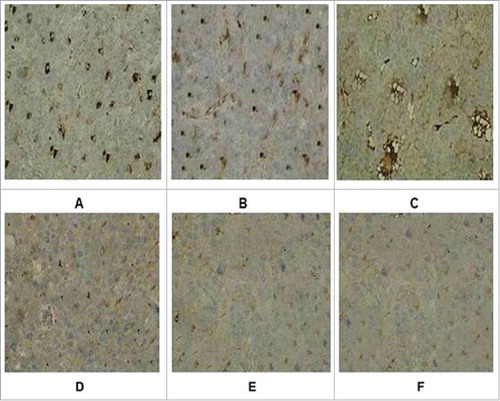
Figure 5. Immunohistochemical staining of tumor tissue in groups. (A) Recombinant adenovirus 5 group. (B)(Adenovirus 5+ LMP2A) group. (C) (Adenovirus 5+ hGM-CSF) group. (D) (Adenovirus 5+ plasmid) group. (E) Adenovirus 5. (F) PBS. The tumor cells in the recombinant adenovirus 5 group compared with the control group, the CD4 cells were more, and in a large number of CD4 in tumor tissue; Adenovirus 5(LMP2A) and Adenovirus 5 (hGM-CSF) group can also be seen with a certain extent part of tumor tissue necrosis and some of CD4 cells, but CD4 cells were less than recombinant adenovirus 5 group, there is no significant difference in the staining results on adenovirus 5 and (adenovirus 5+ plasmid) group. PBS had no tumor necrosis and CD4 cells.