Figures & data
Figure 1. Disposition of participants in the study. 2225 participants were included and randomized 2:2:2:1:1 to receive a single dose of one of the three lots of the 2014–2015 formulation of IIV4, IIV3-1, or IIV3-2. IIV4 contained the A(H1N1), A(H3N2), B Victoria lineage, and B Yamagata lineage strains; IIV3-1 contained the two A strains and the B Victoria lineage strain (IIV3-1); IIV3-2 was the 2014–2015 licensed IIV3 and contained the two A strains and the B Yamagata lineage strain. All but three participants were vaccinated. Reasons for discontinuation included a severe adverse event (SAE), voluntary withdrawal not for an adverse event (AE), noncompliance with the study procedures, and loss to follow-up (LFU). The high number of protocol violations in the IIV3-1 group after day 21 and before month 6 was due to 47 participants who were offered and accepted to receive a second vaccination with the commercial vaccine (IIV3-2) so that they would be covered for the B/Yamagata-lineage strain.
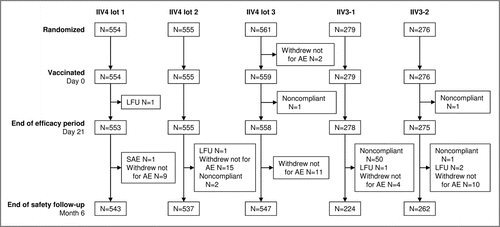
Table 1. Participant baseline characteristics.
Table 2. HAI antibody responses.
Figure 2. Persistence of hemagglutination inhibition (HAI) antibody titers up to 1 year after vaccination with the quadrivalent inactivated influenza vaccine (IIV4). HAI geometric mean antibody titers (GMTs) for younger adult (18–60 years) and older adult (>60 years) participants vaccinated with IIV4 are shown at baseline (day 0), the end of the primary efficacy assessment period (day 21), the end of the safety follow-up period (month 6), and month 12. Values are for all subjects vaccinated and with data available.
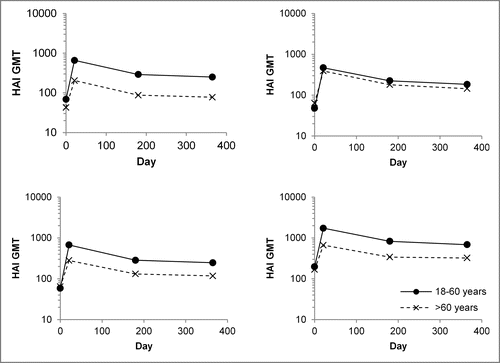
Table 3. Non-inferiority and superiority of post-vaccination (day 21) HAI antibody responses for pooled quadrivalent vs. trivalent vaccines.
Table 4. IIV4 lot equivalence.
Table 5. HAI GMTs at baseline and day 21 by previous year's vaccination.
Table 6. Post-vaccination (day 21) seroprotection rates by previous year's vaccination.
Table 7. SN antibody responses.
