Figures & data
Table 1. Characteristics of Currently Licensed Influenza Vaccines.
Table 2. Serologic Assays Used to Assess Influenza Vaccine Responses.
Table 3. Virus Reagents for HI assay (CP-Q14VLP-009 and CP-Q14VLP-010) in Clinical Trials.Footnote*
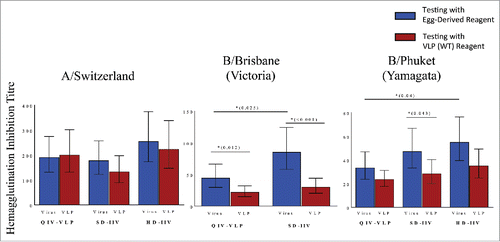
Figure 1. Post-hoc analysis of reagent impact on HAI results in phase II study of a plant-made virus-like particle (VLP) vaccine in elderly subjects ≥65 years of age. Subjects received a single intramuscular dose of a quadrivalent VLP vaccine (QIV-VLP) containing 30mg of each hemagglutinin (HA) used in the 2015-16 seasonal vaccine, a standard dose quadrivalent inactivated vaccine (SD-IIV) containing 30mg of each HA or a high-dose trivalent IIV (HD-IIV) containing 60mg of each HA. Geometric mean titres (GMT) of serum hemagglutination Inhibition (HI) titres were measured in samples from 50 subjects in each arm of the trial using either VLPs (wild-type HA sequence) or reference (egg-derived) reagents in the assay.