Figures & data
Figure 1. The ventral sites of rats were wiped and injected ID with 100 µL of 10% ink-PBS using the MP-0.1 system. The animals were euthanized, and their skin was dissected and lifted to confirm the accuracy of the injection (A, B). On day 0, the rats were immunized IM (syringe) or ID (MP-0.1 system) with 3, 9, or 15 µg of HA, or PBS, in a total volume of 100 µL, and body weight changes were recorded daily (C).
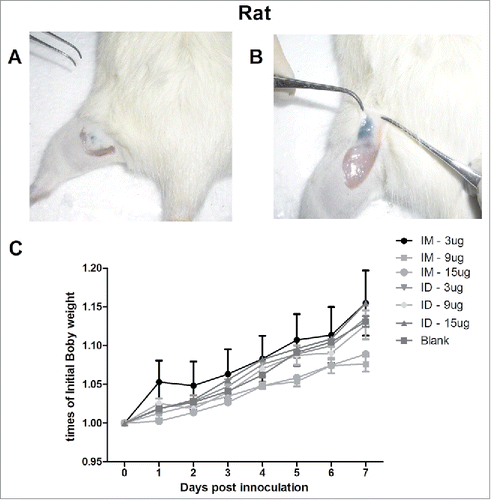
Figure 2. Six-to-eight-week-old BALB/c mice (n = 6) and SD rats (n = 6) were twice immunized ID or IM with 3, 9, or 15 µg of HA of the split H7N9 influenza vaccine at 2-week intervals, and serum samples were collected at 2, 3, and 4 weeks post-boosting for HI antibody measurements (A, B). Mice and rats were immunized with the H7N9 influenza vaccine at 0 and 14 days, and serum HI antibody titers and IgG subtypes were assayed at 2 and 4 weeks post-boosting (C).
Figure 3. BALB/c mice (n = 6) were immunized IM or ID with 3 or 15 µg HA of the H7N9 influenza vaccine, and serum HI antibody titers were measured beginning at day 0 (A). Thirty rats were injected with 3 µg of HA of the H7N9 influenza vaccine, or saline as a control, using a syringe or the MP-0.1 system, and skin biopsies were obtained at 0, 1, 2, 3, and 7 days for assessment of inflammatory cell infiltration into the dermis and muscular layer (B).
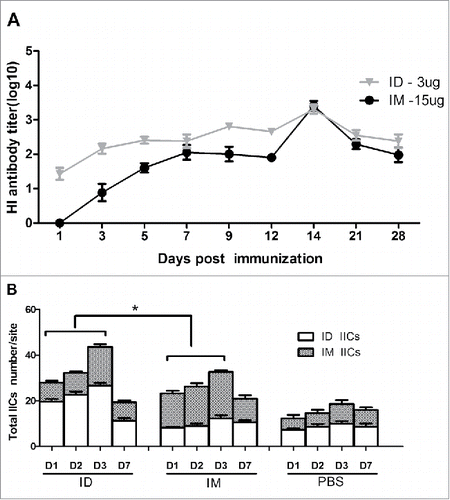
Figure 4. The H7N9 split vaccine was labeled with Cy5 for visualization of antigen deposition and metabolism in vivo. Rats were immunized ID using MP-0.1 or IM using a syringe and anesthetized with 4% isoflurane. Fluorescence signals were visualized at 1 hour, 1 day, 2 days, 5 days, and 7 days, and background auto-fluorescence was subtracted (A). Fluorescence intensity over time (B). At 1, 24, and 48 h, three rats per group were euthanized, and the kidney, liver, spleen, and thymus were removed and subjected to fluorescence imaging (C, D, E, F).
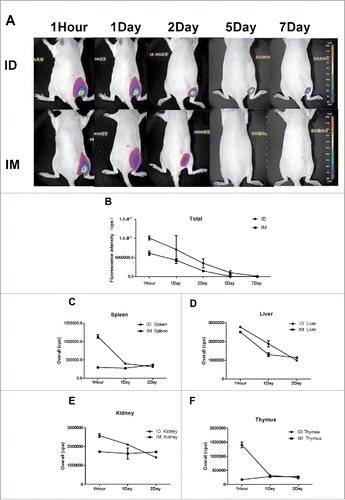
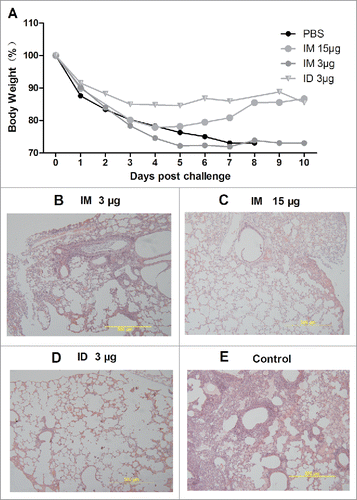
Figure 5. At 0 and 14 days, BALB/c mice were immunized IM with PBS or 15 or 3 µg HA, or they were immunized ID with 3 µg HA of the influenza H7N9 split vaccine. Two weeks later, they were challenged IN with a lethal dose of wild-type A/AnHui/1/2013 H7N9 virus (5.0 × 103 TCID50 pfu). Body weight was monitored daily (n = 6), and pathological changes (n = 3) in the lungs were analyzed at 7 days after challenge (A, B, C, D, E).