Figures & data
Figure 1. Study flow per co-administration vaccination regimen from week 0 until month 26.Group R1 received RTS,S/AS01 + (DTaP/Hib + tOPV + PHiD-CV), and HRV 2 weeks later, Group R2 received RTS,S/AS01 + (DTaP/Hib + tOPV + HRV), and PHiD-CV 2 weeks later, Group R3 received RTS,S/AS01 + (DTaP/Hib + tOPV), and (PHiD-CV + HRV) 2 weeks later, Group C1 received HBV + (DTaP/Hib + tOPV + PHiD-CV), and HRV 2 weeks later, Group C2 received HBV + (DTaP/Hib + tOPV + HRV), and PHiD-CV 2 weeks later, * Other: five consent withdrawal, two recruitment target reached in SBIR, one down syndrome, and one end of inclusion (recruitment was completed), ** Protocol violation: three screening expired and one child received recommended vaccines before enrolment, DTaP/Hib = diphtheria-tetanus-acellular-pertussis-Haemophilus influenzae type-b-conjugate vaccine, tOPV = trivalent oral poliovirus vaccine, PHiD-CV = 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine, HRV = human rotavirus vaccine.
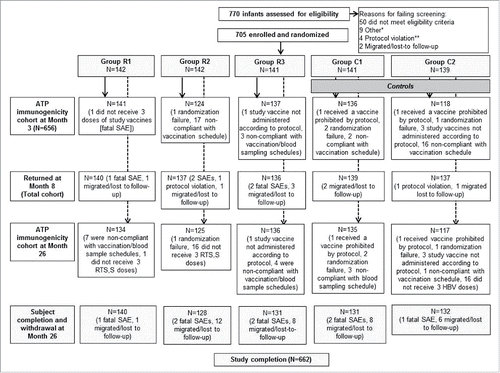
Table 1. Treatment groups.
Table 2. Results of the inferential analyses for HBV, PHiD-CV, HRV and pertussis vaccine antigens administered as three priming doses during infancy (according to protocol immunogenicity cohort).
Table 3. Anti-HBs seroprotection rates and geometric mean antibody titers one month post-dose 3 (ATP immunogenicity cohort).
Table 4. Anti-CS antibody seropositivity rates and geometric mean concentrations (GMC) one month post-dose 3 (adaptedFootnote* according to Protocol immunogenicity cohort).
Figure 2. Anti-pneumococcal antibody concentrations and OPA titers after primary vaccination with PHiD-CV (with or without RTS,S/AS01 co-administration) and booster vaccination (according to protocol immunogenicity cohort at each time point). Vertical lines indicate 95% confidence intervals, OPA = opsonophagocytic activity, PHiD-CV = 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine, GMC/T = geometric mean antibody concentration/titer.
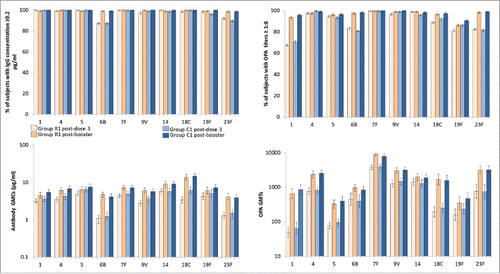
Table 5. Anti-rotavirus antibody seropositivity rates and geometric mean antibody concentrations one month post-dose 2 (according to protocol immunogenicity cohort).
Table 6. Solicited local (by administered product and at any injection site) and systemic symptoms over 7 days post-primary vaccination (Days 0–6) overall doses (Total vaccinated cohortFootnote*).