Figures & data
Table 1. Patients' characteristics at allocation
Figure 2. Differences in the response rates of 23F between the 2 groups (the rate in the simultaneous group minus that in the sequential group)
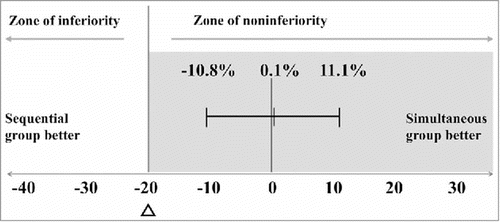
Table 2. Odds ratios for seroresponses 4–6 weeks post-vaccination with the 23-valent pneumococcal polysaccharide vaccine.
Table 3. Antibody titers to pneumococcal capsular polysaccharides.
Table 4. Odds ratios for seroprotection 4–6 weeks post-vaccination with the quadrivalent influenza vaccine.
Table 5. Adverse events in patients of the simultaneous and sequential groups.