Figures & data
Figure 1. Participant flowchart. Footnote: Td group, participants receiving Td as first booster dose in the primary study and Tdap as decennial booster dose (second booster dose) in the current study; Tdap group, participants receiving Tdap booster doses 10 years apart; N, number of participants in each group; M, month; ATP, according-to-protocol.
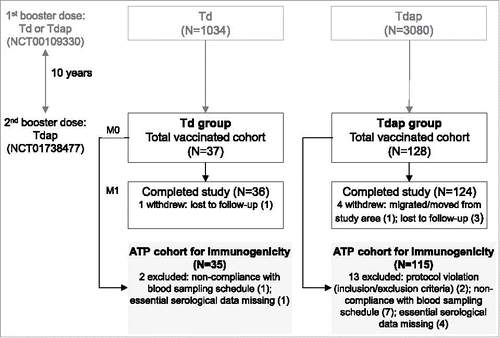
Table 1. Summary of demographic characteristics (total vaccinated cohort).
Table 2. Results of co-primary objectives.
Table 3. Immune responses to Tdap vaccination (according-to-protocol cohort for immunogenicity).
Table 4. Booster response and alternative booster response to diphtheria and tetanus and booster response to pertussis components, overall and by initial serostatus (according-to-protocol cohort for immunogenicity).
Table 5. Summary of reactogenicity and safety following administration of a Tdap dose (total vaccinated cohort).