Figures & data
Figure 1. Preclinical testing of the VSV-EBOV vaccine in animal models. Recombinant VSV particles expressing the EBOV GP are produced from a cDNA clone of the VSV genome in which the VSV G is replaced with EBOV GP. The resulting vaccine has been tested in different animal models to assess protective prophylactic efficacy, time to immunity, post-exposure efficacy, cross-protection potential, as well as providing insight into the mechanism of protection.
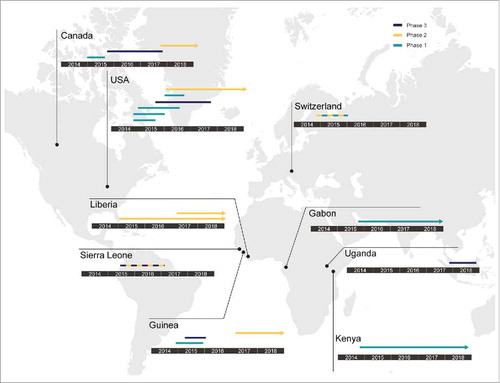
Figure 2. Concluded, ongoing and planned human clinical trials of VSV-EBOV. Since 2014, VSV-EBOV has been evaluated globally in phase 1, 2 and 3 clinical trials. The countries where these clinical trials were conducted, and their phase (1-3) are shown. Lines indicate completed clinical trials; arrows indicate clinical trials still ongoing at the time of this writing.