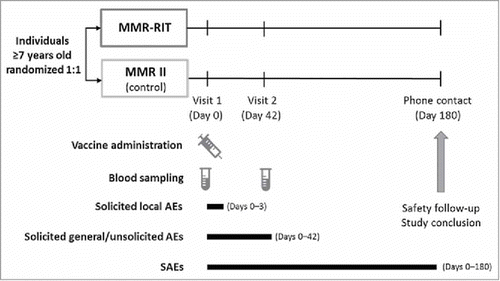
Figures & data
Figure 2. Flow diagram of the study participants. Footnote: N, number of participants; n, number of participants within the category; GCP, good clinical practice; TVC, total vaccinated cohort; ATP, according-to-protocol.
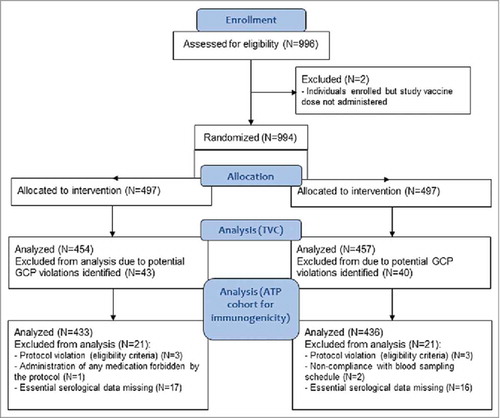
Table 1. Demographic characteristics of the study participants (total vaccinated cohort, N = 911).
Table 2. Non-inferiority of MMR-RIT vaccine compared to MMR II in terms of anti-measles, anti-mumps and anti-rubella adjusted geometric mean antibody concentrations at Day 42 (ATP cohort for immunogenicity).
Table 3. Non-inferiority of MMR-RIT vaccine compared to MMR II in terms of anti-measles, anti-mumps and anti-rubella seroresponse rates at Day 42 (ATP cohort for immunogenicity).
Figure 3. Percentage of participants who achieved a 4-fold or greater increase in anti-measles, anti-mumps, or anti-rubella virus antibody concentrations at Day 42 (ATP cohort for immunogenicity). Footnote: N, number of participants with both pre- and post-vaccination available results; ATP, according-to-protocol.
For participants with a seronegative status at pre-vaccination, a 4-fold rise in antibody concentration is defined as 4 times the cut-off level of the assay. Cut-off levels for anti-measles, anti-mumps and anti-rubella virus antibody concentrations are 150 mIU/mL, 5 EU/mL and 4 IU/mL, respectively. The error bars represent the upper and lower limits of the two-sided 95% confidence intervals obtained using the Clopper Pearson method.
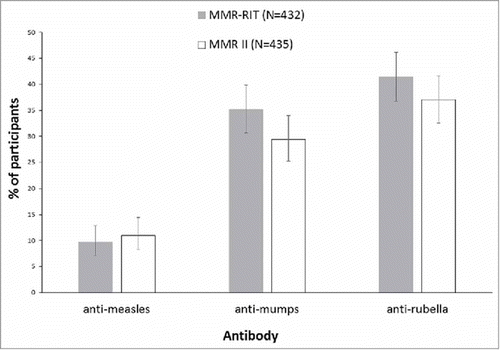
Figure 4. Incidence of solicited injection site (Day 0–3) and general adverse events (Day 0–42) (total vaccinated cohort). Footnote: N, number of participants with the documented dose with local symptoms sheets completed *Except for pain, redness, and swelling, for which MMR-RIT (N = 433). Fever: temperature ≥38°C. Grade 3 was defined as: limb was painful at rest, which prevented normal everyday activities (pain); diameter >50 mm (redness and swelling); temperature >39.5°C (fever); adverse event preventing normal, everyday activities (joint pain, rash/exanthem, meningism/seizure); swelling with accompanying general symptoms (parotid/salivary gland swelling). The error bars represent the upper and lower limits of the two-sided 95% confidence intervals obtained using the Clopper Pearson method.
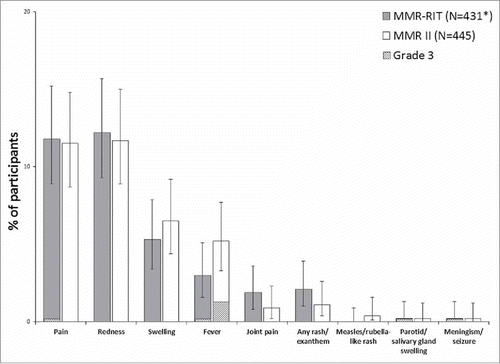
Table 4. Percentage of participants with unsolicited adverse events (Day 0–42) and serious adverse events (Day 0–180) (total vaccinated cohort).