Figures & data
Table 1. Baseline characteristics of commercial/Medicare and of Medicaid enrollees who meet study selection criteria, by type of vaccine at initial vaccination dose.
Figure 1. Rates of vaccination series completion among (a) adult commercial/Medicare (N = 350,240) and (b) adult Medicaid patients (N = 12,599)*
*Schedule of vaccine series was determined by type of vaccine (monovalent hepatitis A or B or bivalent hepatitis A/B) administered on index date. Completion schedules with a bivalent vaccine on the index date are shown stratified by whether series fulfilled hepatitis A or B coverage; Hepatitis A vaccine is a 2-dose series; however, discrepancies in series completion for hepatitis A allow for bivalent vaccine to be included in the measurement, thus needing three doses, as appropriate, to be complete. The bivalent inclusion required 3 doses for completion.
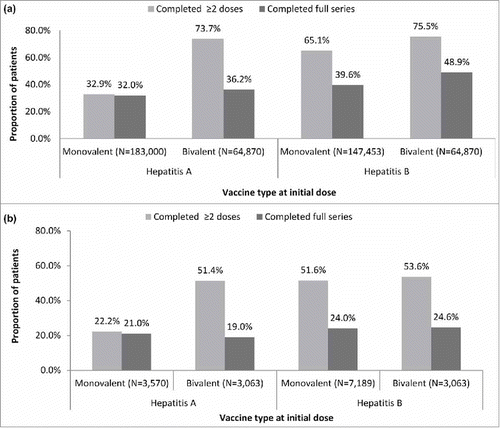
Figure 2. Study design scheme; Abbreviations: CPT, Current Procedural Terminology
*Hepatitis A adult dosage, CPT 90632; Hepatitis B adult standard 3 doses, CPT: 90740; Hepatitis B for dialysis or immunocompromised patients, 3 doses, CPT: 90746; Hepatitis B for dialysis or immunocompromised patients, 4 doses, CPT: 90747; Hepatitis A and B adult dosage, CPT: 90636.
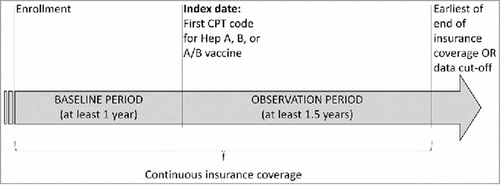
Table 2. Hepatitis A, B, or A/B Vaccine Completion Adult Schedule GuidelinesCitation11,Citation12
