Figures & data
Figure 1. Incidence rate of IPD in Hong Kong children according to age groups. Since September 2009, all children were immunized using a 3-dose primary series at 2, 4 and 6 months of age and a booster dose at age 12–15 months. The incidence rates (as 100,000 per persons per year) were grouped into four periods to indicate the burden before availability of PCV (period 1, 1995–2004), availability in the private market (period 2, 2006–2009), and following early (period 3, 2010–2014) and more than 5 years (period 4, 2015–2017) of implementation in the childhood immunization program. Differences in the rates in the time periods were assessed by chi-square for trend. §P < 0.001.
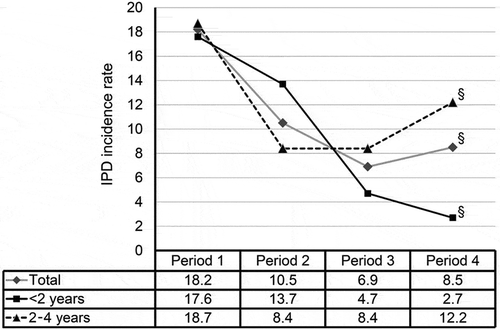
Figure 2. Incidence rate of IPD in Hong Kong children according to serotype groups. Since September 2009, all children were immunized using a 3-dose primary series at 2, 4 and 6 months of age and a booster dose at age 12–15 months. The incidence rates (as 100,000 per persons per year) were grouped into four periods to indicate the burden before availability of PCV (period 1, 1995–2004), availability in the private market (period 2, 2006–2009), and following early (period 3, 2010–2014) and more than 5 years (period 4, 2015–2017) of implementation in the childhood immunization program. Differences in the rates in the time periods were assessed by chi-square for trend. §P < 0.001, *P = 0.369.
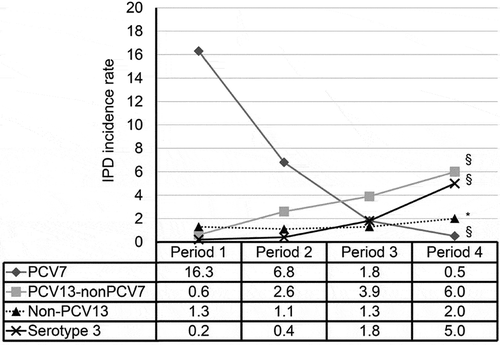
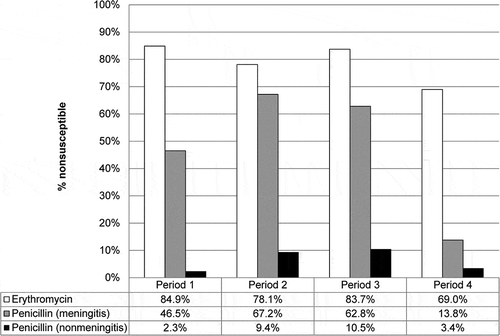
Figure 3. Antimicrobial resistance rates for IPD isolates in different time periods in Hong Kong. Since September 2009, all children were immunized using a 3-dose primary series at 2, 4 and 6 months of age and a booster dose at age 12–15 months. The time periods indicate a time before availability of PCV (period 1, 1995–2004), availability in the private market only (period 2, 2006–2009), and following early (period 3, 2010–2014) and more than 5 years (period 4, 2015–2017) of implementation in the childhood immunization program.