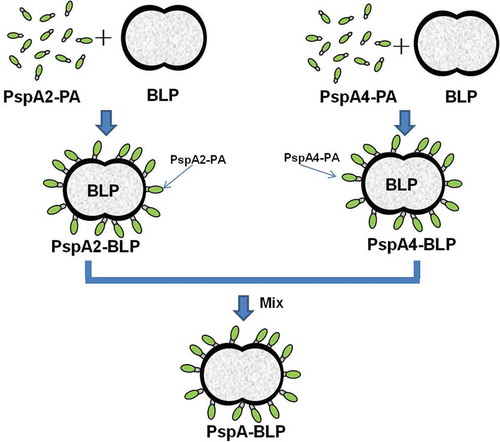
Figures & data
Figure 1. Identification of PspA2-PA and PspA4-PA binding with BLPs. (A) SDS-PAGE analysis of PspA2-PA and PspA4-PA binding with BLPs. Lane 1, protein molecular weight marker; lane 2, PspA2-PA binding with BLP; lane 3, BLP control; lane 4, PspA4-PA binding with BLP. (B) Binding capacity of PspA2-PA and PspA4-PA with BLPs. The amount of proteins binding to the BLPs was calculated by comparison with BSA standards in SDS-PAGE analysis.
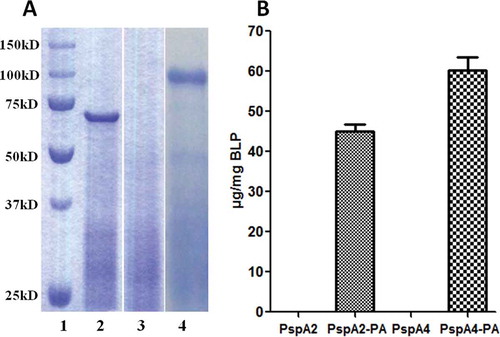
Figure 2. Analysis of antigenicity of PspA2-BLP and PspA4-BLP by confocal immunofluorescence microscopy. (A) PspA2-BLP, BLP. (B) PspA4-BLP, BLP. PspA2-BLP or PspA4-BLP was incubated with anti-PspA2 or anti-PspA4 antibody. BLP as the control and FITC-labeled secondary antibodies were used. The preparations were analyzed using bright field and fluorescence microscopy.
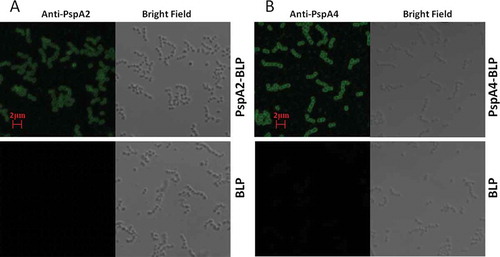
Figure 3. Analysis of immunogenicity of PspA-BLP. (A) PspA2-specific IgG titers in serum. (B) PspA4-specific IgG titers in serum. (C) PspA2-specific SIgA titers in bronchoalveolar washes. (D) PspA4-specific SIgA titers in bronchoalveolar washes. *P < 0.05.
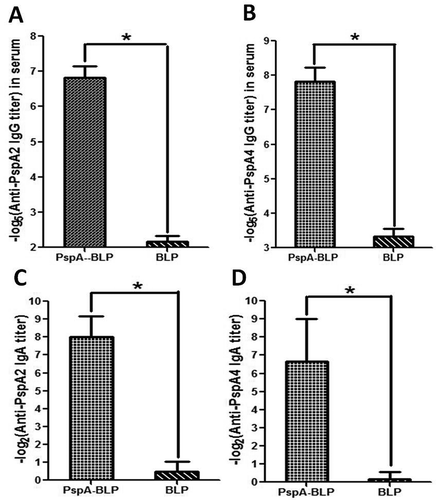
Figure 4. Binding of serum antibodies to intact pneumococci. Sera from mice immunized with PspA-BLP or BLP were tested for the ability to bind to pneumococcal strains expressing PspA from clade 1 (A), clade 2 (B), clade 3 (C), clade 4 (D) and clade 5 (E). Results are shown as fluoresence intensity for each group.
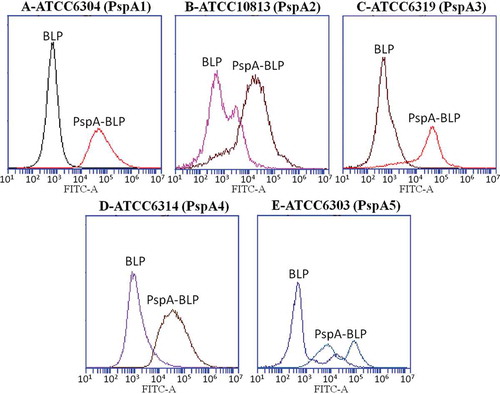
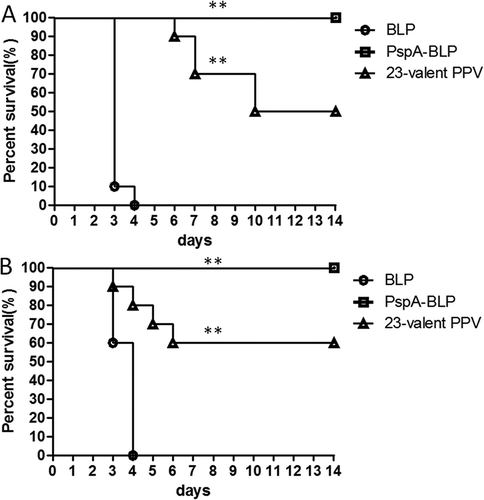
Figure 5. Protection against pneumococcal challenge with strains bearing heterologous PspAs. Mice were intranasally immunized with PspA-BLP pneumococcal vaccine and BLP as negative control. The 23-valent PPV was used as a positive control, which was immunized in mice via the subcutaneous route. Two weeks after the third immunization of PspA-BLP and BLP (six weeks after the first immunization of 23-valent PPV), the immunized mice were challenged intranasally with S. pneumoniae. (A) Immunized mice were challenged with the ATCC10813 pneumococcal strain (PspA family 1). (B) Immunized mice were challenged with the ATCC6303 pneumococcal strain (PspA family 2). Ten mice per group were examined in each challenge experiment. Mortality was monitored for 14 days, and survival curves are shown in the figure. **P < 0.01 versus BLP control group.