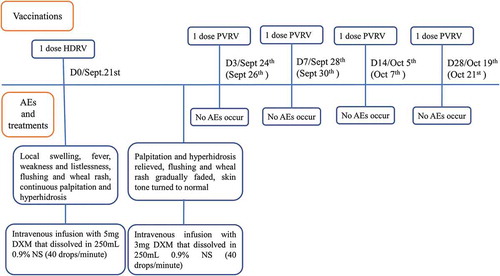
Figures & data
Table 1. Human rabies vaccine marketed in China.
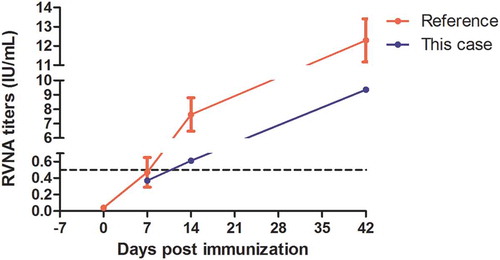
Figure 2. RVNA titers of this case and reference data from a previous studyCitation16 which used the same kind of vaccine with Essen regimen. The black dotted line (RVNA = 0.5 IU/mL) indicated minimum protective level of sero-conversion. Day 7 was actually 9 days post the first dose because of two-days delay on anaphylaxis treatment. It was the third dose in regimen and reflected the immune response of previous two doses, so in this figure, it was marked as day 7. It was comparative to the reference data. As for day 14, it reflected the immune response of previous three doses, though it was 16 days post the first dose, it was the fourth dose (Day 14) in regimen.