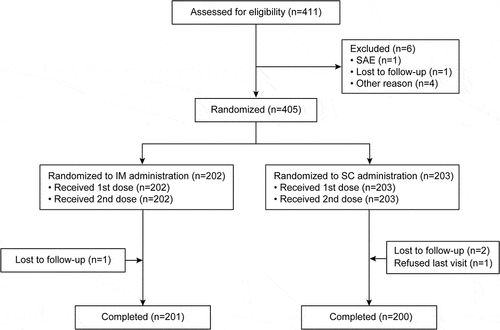
Figures & data
Table 1. Characteristics of overall and antigen-specific per protocol set for primary analyses post dose 2 of MMRV (PPS2).
Table 2. Summary of non-inferiority analyses (stratified by region) of antibody response rates to measles, mumps, rubella, and varicella 6 weeks after the second dose of MMRV for participants initially seronegative to measles, mumps, rubella, and varicella – antigen specific per protocol set post-dose 2 (PPS2).
Table 3. Summary of non-inferiority analyses (stratified by region) of antibody response rates to measles, mumps, rubella, and varicella 6 weeks after the second dose of MMRV – Full Analysis Set (FAS).
Table 4. Summary of GMTs to measles, mumps, rubella, and varicella 4 weeks after the first dose and 6 weeks after the second dose of MMRV for participants initially seronegative to measles, mumps rubella and varicella – Antigen specific per protocol set post-dose 1 (PPS1) and post-dose 2 (PPS2).
Table 5. Summary of GMTs to measles, mumps, rubella and varicella 4 weeks after the first dose and 6 weeks after the second dose of MMRV – Full Analysis Set (FAS).
Table 6. Summary of injection site and systemic adverse events occurring from day 0 to day 28 post-dose 1 and post-dose 2 of MMRV.