Figures & data
Figure 2. Flow diagram of the study participants in each sub-cohort.
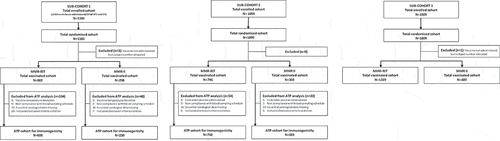
Table 1. Demographic characteristics of the study participants (total vaccinated cohort).
Table 2. Anti-measles, anti-mumps, and anti-rubella seroresponse rates and geometric mean antibody concentrations at Day 42 when MMR-RIT and MMR II were co-administered with DTaP-IPV and VV (according-to-protocol cohort for immunogenicity, sub-cohort 1).
Table 3. Anti-measles, anti-mumps, and anti-rubella seroresponse rates and geometric mean antibody concentrations at Day 42 when MMR-RIT and MMR II were administered alone (according-to-protocol cohort for immunogenicity, sub-cohort 2).
Table 4. Seroresponse rate and booster response rate to antibodies to the co-administered vaccines at Day 42 (according-to-protocol cohort for immunogenicity, sub-cohort 1).
Table 5. Geometric mean antibody concentrations or titers to the co-administered vaccines at Day 42 (according-to-protocol cohort for immunogenicity, sub-cohort 1).
Figure 3. Incidence, in each sub-cohort, of solicited injection site adverse events (Day 0–3), fever (Day 0–42), and drowsiness and loss of appetite (Day 0–3; only assessed in sub-cohort 1) (total vaccinated cohort).
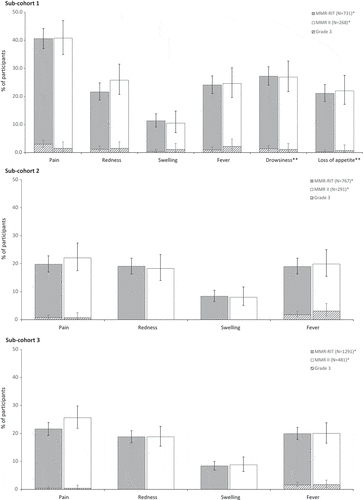
Table 6. Incidence of rash, parotid/salivary gland swelling, and febrile convulsions/headaches (Day 0–42) (total vaccinated cohort).
Table 7. Incidence of reported unsolicited adverse events (Day 0–42), serious adverse events, and NOCDs (Day 0–180) (total vaccinated cohort).
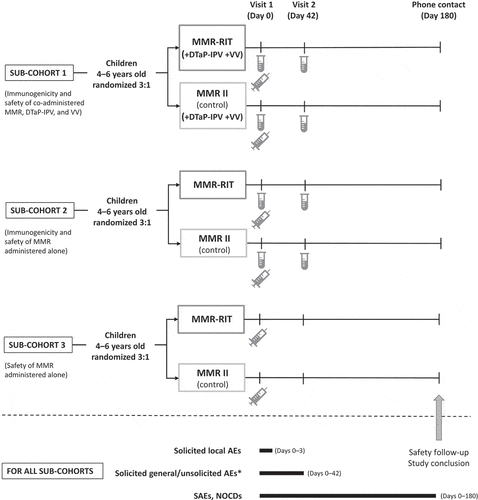
Figure 4. Study design.
 , vaccine administration (includes administration of DTaP-IPV and VV in sub-cohort 1); AEs, adverse events; DTaP-IPV, diphtheria, tetanus, acellular pertussis, and inactivated polio vaccine; NOCDs, new onset chronic diseases; SAEs, serious adverse events; VV, varicella vaccine.*Drowsiness and loss of appetite were recorded as solicited general AE from Day 0 to Day 3 in sub-cohort 1 only.
, vaccine administration (includes administration of DTaP-IPV and VV in sub-cohort 1); AEs, adverse events; DTaP-IPV, diphtheria, tetanus, acellular pertussis, and inactivated polio vaccine; NOCDs, new onset chronic diseases; SAEs, serious adverse events; VV, varicella vaccine.*Drowsiness and loss of appetite were recorded as solicited general AE from Day 0 to Day 3 in sub-cohort 1 only.