Figures & data
Table 1. Demographics of subjects at baseline, total vaccinated cohort (TVC).
Figure 1. Study Flow.
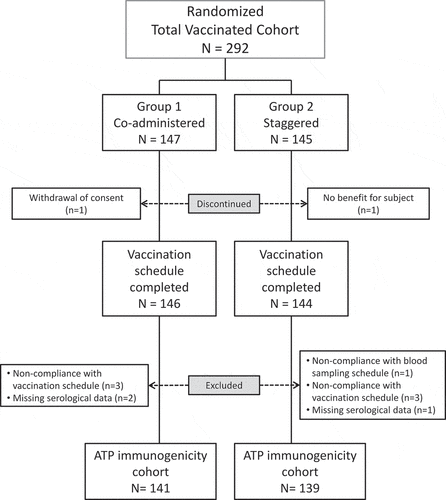
Table 2. Seroprotection rates (SPR) for diphtheria toxin, tetanus toxin, and polio virus type 1, 2 and 3 and seropositivity rates for pertussis (PT, FHA) for co-administered and staggered groups at one month following the third dose of DPT-IPV vaccine (ATP cohort for immunogenicity).
Table 3. Geometric mean titers (GMTs) and concentrations (GMCs) for diphtheria toxin, tetanus toxin, pertussis (PT, FHA), and polio virus type 1, 2 and 3 for co-administered and staggered groups at one month following the third dose of DPT-IPV vaccine (ATP cohort for immunogenicity).
Table 4. Summary of adverse events, total vaccinated cohort (TVC).
Figure 3. Vaccination schedule in the co-administered group and staggered group.
 DPT-IPV vaccine, diphtheria-pertussis-tetanus and inactivated polio vaccine;
DPT-IPV vaccine, diphtheria-pertussis-tetanus and inactivated polio vaccine; 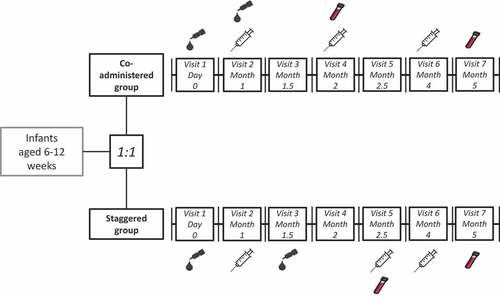
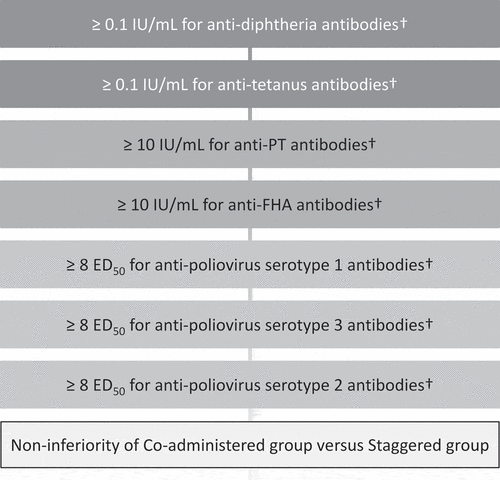
Figure 4. Hierarchical procedure to test the primary confirmatory objective.