Figures & data
Table 1. Study design, vaccines administered, and participant flow.
Figure 1. HAI Antibody response to vaccination with quadrivalent split-virion influenza vaccine. Blood was collected before vaccination (day 0) and 28 days after the last vaccination. Serum hemagglutination (HAI) titers were measured as described previouslyCitation16 in all vaccinated subjects with data available and are expressed as 1/dilution. HAI titers under the lower limit of quantitation (10) were assigned a value of 5, and all HAI titers above the upper limit of quantitation (10,240) were assigned a value of 10,240. (A) HAI geometric mean titers (GMTs) and 95% confidence intervals (CIs) were determined from log10-transformed data using Student’s t-distribution with n−1 degrees of freedom, after which antilog transformations were applied to the results of calculations. (B) Geometric mean ratio of the individual post-/pre-vaccination HAI titer ratio (GMTR). (C) Proportion of participants seroconverting or with a significant increase in titer. Seroconversion was defined as a pre-vaccination HAI titer < 10 and a post-vaccination HAI titer ≥ 1:40. A significant increase was defined as a pre-vaccination HAI titer ≥ 10 and a ≥ 4-fold increase in HAI titer between pre- and post-vaccination. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
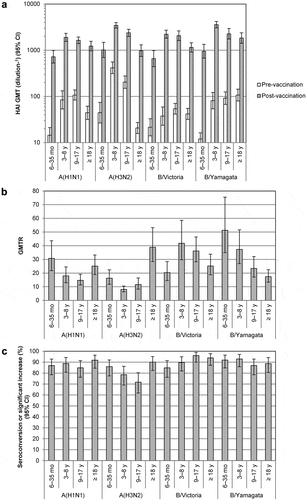
Table 2. Solicited reactions.
