Figures & data
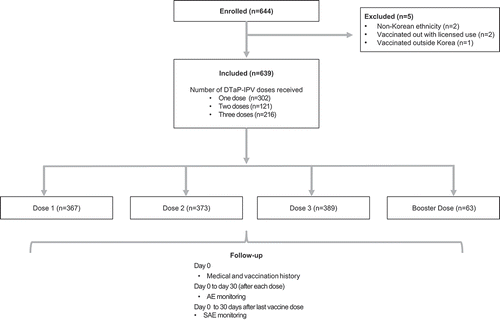
Table 1. Study population and vaccine doses received.
Table 2. Adverse events (AEs) and adverse drug reactions (ADRs) over the PMS period.
Table 3. Unexpected adverse events and adverse drug reactions over the PMS period.
Table 4. Serious adverse events and serious adverse drug reactions over the PMS period.
Table 5. Subject characteristics and incidence of adverse events (AEs).