Figures & data
Figure 1. Flow chart of subject enrollment in the test-negative design case-control study for the estimates of EV-A71 vaccine effectiveness during 2017, in Beijing, China.
Note: HFMD: hand, foot, and mouth disease. EV-A71: enterovirus A71. BMSIIP: Beijing Management System of Information for the Immunization Program. VE: vaccine effectiveness.
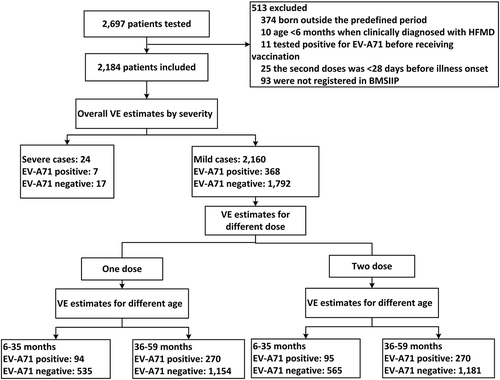
Table 1. Comparison of demographic characteristics and virus infection between mild vaccinated and non-vaccinated HFMD cases.
Figure 2. Monthly number of the cases testing negative and positive for enterovirus by serotype.
Note: EV-A71: enterovirus A71. CV-A16: coxsackievirus A16. CV-A6: coxsackievirus A6. Others: otherenterovirus than EV-A71, CV-A16 and CV-A6. Negative: negative for all enteroviruses.
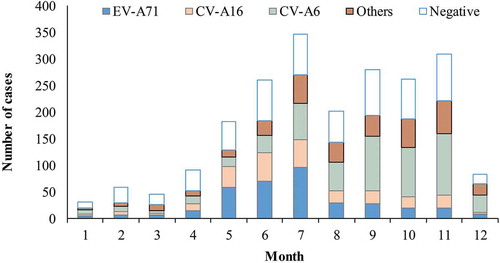
Figure 3. Timeline of number of subjects receiving two doses of EV-A71 vaccine.
Note: EV-A71: enterovirus A71. The number of subjects who received EV-A71 vaccine were excluded: (1) who only received one dose of EV-A71 vaccine, and (2) who received a second dose of EV-A71 vaccine that was <28 days before the illness onset.
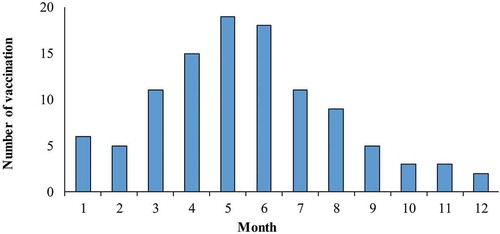
Table 2. Comparison of demographic characteristics and virus infection between mild EV-A71-positive cases and EV-A71-negative controls.
Table 3. Crude and adjusted estimates of vaccine effectiveness against mild, medically-attended EV-A71-HFMD for different number of doses and age group.
Table 4. Estimates of vaccine effectiveness in sensitivity analysis, using controls who were positive for an enterovirus other than EV-A71.
Table 5. Estimates of vaccine effectiveness in sensitivity analysis, using pan-EV negative controls.
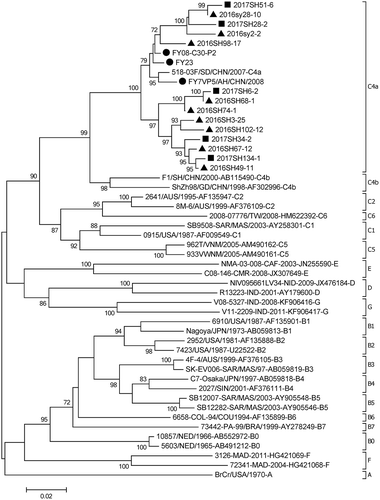
Figure 4. Phylogentic analysis of VP1 gene of EV-A71 strains from HFMD virological surveillance during the 2016–2017 in Beijing, China#.
Note: EV-A71: enterovirus A71.HFMD: hand, foot, and mouth disease.# The EV-A71 strains analyzed in this study were indicated with solid triangles and squares, and the vaccine strains were shown with solid dots. ▲the strains isolated in 2016, ■ the strains isolated in 2017; ● vaccine strains