Figures & data
Figure 1. Schematic illustrating serum bactericidal antibody assay using either human or baby rabbit complement. For serogroups A and C, the World Health Organization guidelines stipulate that complement sourced from baby rabbits should be used.Citation24 For the subject shown, the SBA titer would be 8. SBA = serum bactericidal antibody. Figure has been adapted with permission from Gandhi A, Balmer P, York LJ. Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba®). Postgrad Med. 2016;128(6):548–556
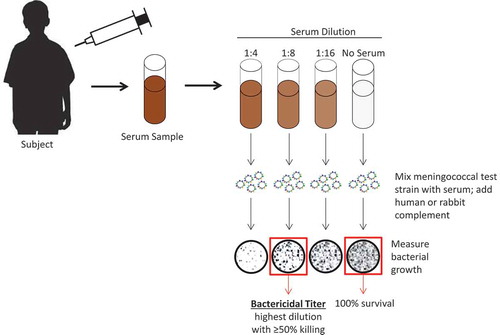
Figure 2. Percentages of infants/toddlers with rSBA titers ≥8 or hSBA titers ≥4 for serogroups A, C, W, and Y at various time points after vaccination with either 3 primary doses of MenACWY-TT (at 2, 4, and 6 months of age) followed by a booster dose at 15–18 months of age (3 + 1 schedule) or 1 primary dose of MenACWY-TT at 6 months of age followed by a booster dose at 15–18 months of age (1 + 1 schedule). Data are plotted as percentages along with 95% CIs. hSBA = serum bactericidal antibody assay using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y conjugate vaccine using tetanus toxoid as a carrier protein; rSBA = serum bactericidal antibody assay using rabbit complement. Data are from Dbaibo G, Tinoco Favila JC, Traskine M, Jastorff A, Van der Wielen M. Immunogenicity and safety of MenACWY-TT, a meningococcal conjugate vaccine, co-administered with routine childhood vaccine in healthy infants: a phase III, randomized study. Vaccine. 2018;36(28):4102–4111
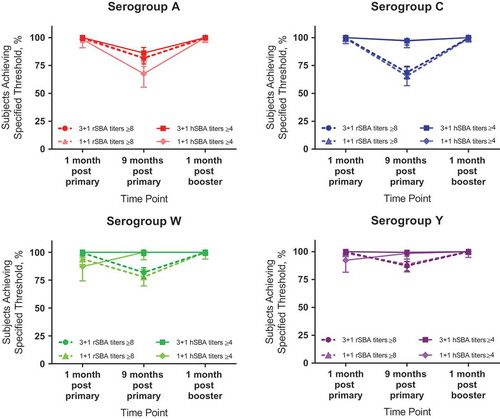
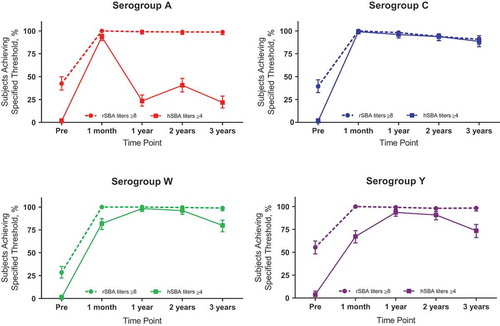
Figure 3. Percentages of toddlers with rSBA titers ≥8 or hSBA titers ≥4 for serogroups A, C, W, and Y at various time points before or after vaccination with 1 dose of MenACWY-TT at 12 to 23 months of age. Data are plotted as percentages along with 95% CIs. hSBA = serum bactericidal antibody assay using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y conjugate vaccine using tetanus toxoid as a carrier protein; pre = prevaccination; rSBA = serum bactericidal antibody assay using rabbit complement. Data are from Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–1903