Figures & data
Table 1. Batch release specifications for GRAZAX®
Figure 1. Scheme of the immunological response to allergen-specific immunotherapy (AIT). AIT during the early phase of the treatment (1–4 months), induces both mast cell desensitization and upregulation of Th2 response, mediated by high levels of IgE and IL4. During the active phase (1–3 y), a switch of isotype occurs. The levels of IgG4 increase while IgE significantly decrease. In this period is the maximum effect of IgG4 interference. Later, after 3 y of AIT treatment, in the post-therapy period, the regulatory response is established, and levels of IgE and IL4 are significantly decreased
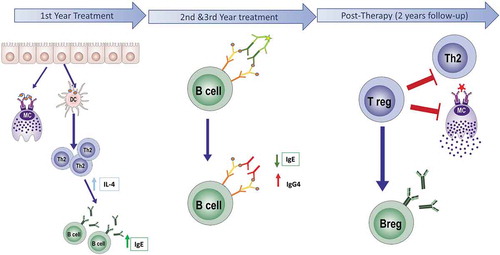
Figure 2. Example of a 5-y prospective clinical trial design
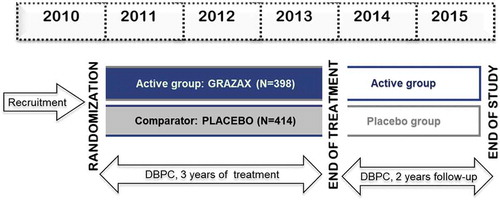
Figure 3. Clinical outcome of pivotal 5-y study
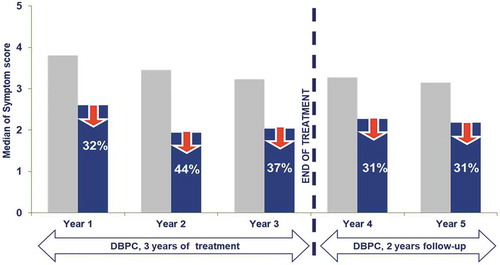
Figure 4. Summary of clinical trials performed in Europe
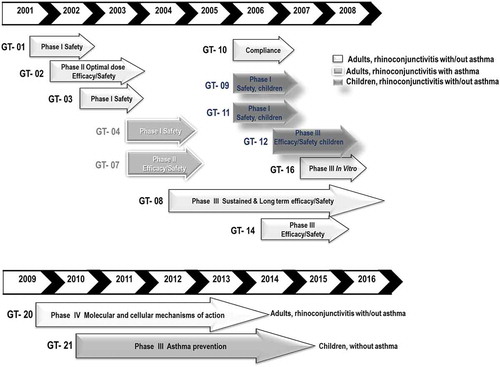
Table 2. Summary of main Clinical trials performed with GRAZAX®
