Figures & data
Figure 1. Study design flow chart with the disposition of subjects from the primary study to follow-up studies. ATP, according-to-protocol; HPV: Human Papillomavirus; n: number of seropositive subjects; N: number of tested subjects; TVC, total vaccinated cohort.
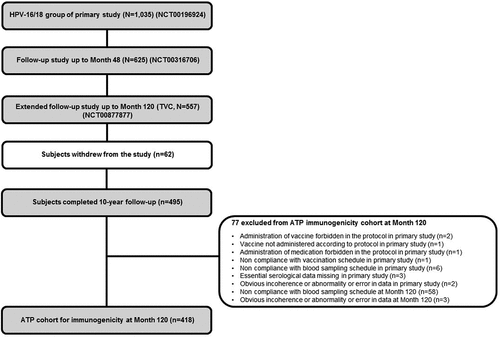
Figure 2. GMTs for anti-HPV-16 and anti-HPV-18 antibodies in initially seronegative subjects (Month 120 ATP immunogenicity cohort). CI: 95% Confidence interval; ELISA: Enzyme-linked immunosorbent assay; EU: ELISA units; GMT: Geometric Mean Titer; HPV: Human papillomavirus; M: Month.
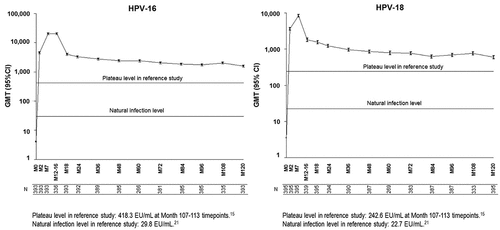
Figure 3. GMTs for anti-HPV-31 and anti-HPV-45 antibodies in initially seronegative subjects (Month 120 ATP immunogenicity cohort). CI: 95% Confidence interval; ELISA: Enzyme-linked immunosorbent assay; EU: ELISA units; GMT: Geometric Mean Titer; HPV: Human papillomavirus; n: number of seropositive subjects; N: number of tested subjects.
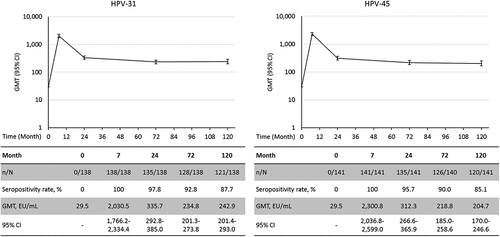
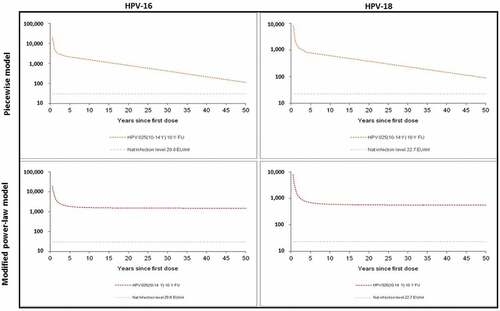
Table 1. Predicted duration ensuring 95% of women have HPV-16 and -18 antibody titers above antibody titers after natural infection. ELISA: enzyme-linked immunosorbent assay; EU: ELISA unit; GMT: geometric mean titer; HPV: human papillomavirus.