Figures & data
Figure 2. Flow of participants
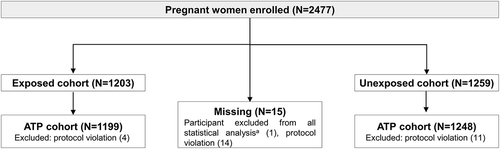
Table 1. Demographic characteristics of study participants (total-enrolled cohort)
Table 2. Cumulative incidence of pregnancy-related adverse events and neonatal AEs of interest in current pregnancy in the exposed and unexposed cohorts (total-enrolled cohort)
Table 3. Estimated coefficients for the adjusted logistic regression model to explore the risk factors of pregnancy-related AEs and neonatal AEs of interest in current pregnancy (Total cohort)
Table 4. Cumulative incidence of other pregnancy-related adverse events and neonatal AEs of interest in current pregnancy in the exposed and unexposed cohorts (total-enrolled cohort)