Figures & data
Table 1. Baseline characteristics of participants (total vaccinated cohort)
Figure 1. Flow chart for the recruitment of volunteers in the study. Titers for antibody to MenA≥1:8 pre-vaccination were two participants in the one and two doses group, respectively. Titers for antibody to MenC ≥1:8 pre-vaccination were three participants in the one and two doses group, respectively. They were removed from the analysis set (mPPS) for immunogenicity
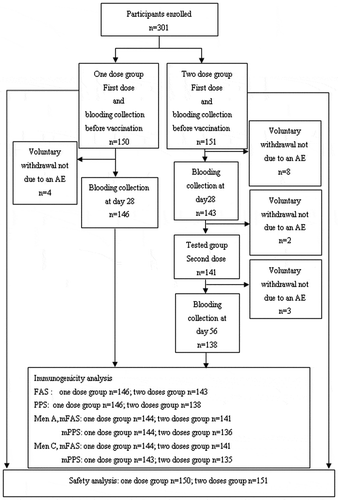
Table 2. Percentage of participants with rSBA titers≥1:8 and ≥1:128 and geometric mean titers against the serogroups MenA and MenC (mPPS cohort for immunogenicity)
Table 3. Common symptoms analysis of solicited local and systemic ARs occurring after vaccination
