Figures & data
Table 1. Demographic characteristics (pooled ZOE-50/70 modified Total Vaccinated Cohort)
Figure 1. Participant flow (pooled ZOE-50/70 studies) RZV = participants receiving the adjuvanted recombinant zoster vaccine; Placebo = participants receiving placebo; ZOE-50/70 = RZV efficacy studies in adults ≥50 YOA (NCT01165177) and ≥70 YOA (NCT01165229), respectively; (m)TVC = (modified) total vaccinated cohort; HZ, herpes zoster
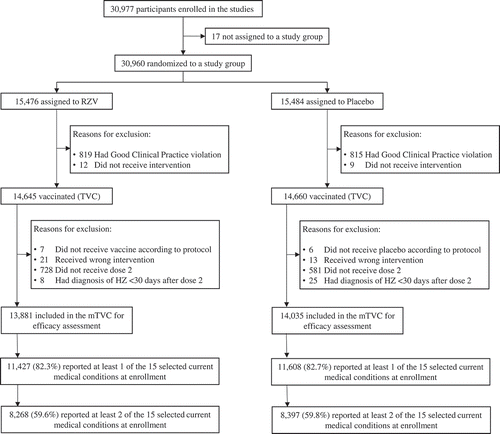
Table 2. Vaccine efficacy against first or only episode of herpes zoster in ZOE-50/70 participants reporting at least 1 of the 15 selected medical conditions at enrollment (pooled modified Total Vaccinated Cohort)
Table 3. Safety of RZV in ZOE-50/70 participants reporting at least 1 of the 15 selected medical conditions at enrollment (pooled Total Vaccinated Cohort)
