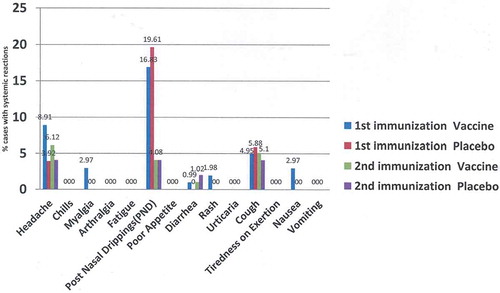
Figures & data
Table 1. Baseline characteristics of screen-failed and enrolled participants in the phase II trial conducted in Thailand
Figure 1. Adverse events by systemic organ classes (%by each group) either receiving H5N2 LAIV vaccine or placebo
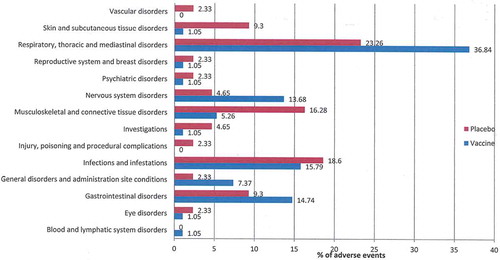
Table 2. Comparing seroconversion rates of 4 fold-rising between previously vaccinated with LAIV H5N2 and naïve subjects by hemagglutinin-inhibition assay (HAI) at day 1, 7, 28 and 90 (Test Virus: A/17turkey/05/133 (H5N2))
Table 3. Comparing seroconversion rates of 4 fold rising between previously vaccinated with LAIV H5N2 and naïve subjects by hemagglutinin-inhibition assay (HAI) at day 1, 7, 28 and 90 (Test virus: rg-H5N1-KAN-1)
Table 4. Geometric mean of immune response by previously vaccinated with LAIV H5N2 and naïve subjects