Figures & data
Figure 1. Flow chart demonstrating stratified randomization for intradermal (ID) and intramuscular (IM) injection of the 2011–2012 vaccine in chronic obstructive pulmonary disease (COPD) patients.
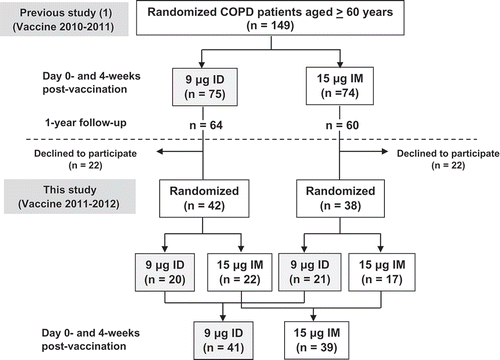
Table 1. Baseline characteristics of COPD patients in the intradermal (ID) and intramuscular (IM) groups.
Table 2. The hemagglutination inhibition (HI) titres† of pre-vaccination and 4 weeks post-vaccination of COPD patients in the intramuscular (IM) group compared between BMI < 18.5 kg/m2(n = 15) and BMI ≥ 18.5 kg/m2(n = 24).
Table 3. Hemagglutination inhibition (HI) antibody titres pre- and 4 weeks post-vaccination of repeated identical influenza virus strains (vaccine 2011–2012) in the intradermal (ID, n = 41) and intramuscular (IM, n = 39) groups.
Table 4. Hemagglutination inhibition (HI) antibody titres† pre- and 4 weeks post-vaccination of annual repeated identical influenza virus strains (2011–2012 vaccine) and previous study (vaccine 2010–2011 vaccine)Citation9 in the intradermal (ID) and intramuscular (IM) groups.
Table 5. Geometric mean titres (GMTs) and seroprotection rates for influenza A(H1N1)pdm09 and influenza A(H3N2) at baseline and 4 weeks, 3 months, 6 months, and 12 months post-vaccination in the ID and IM groups.
Figure 2. (a)Geometric mean titres (GMTs) with SD and (b) seroprotection rates (HI titre ≥ 1:40) at pre-vaccination,4 weeks,3 months, 6 months, and12tmonths post-vaccination in the ID and IM groups of COPD patients aged >60 years. Numbers inthe ID group: at pre-vaccination = 41,4weeks = 41,3 months = 37, 6months = 37,12months = 35. Numbers in the IM group: at baseline = 39, 4weeks = 39, 3 months = 34, 6months = 36, and 12months = 32.(a) Reference line is the seroprotective HI titre ≥ 40; (b) reference line is the CPMP criterion of seroprotection rate >60% for patients aged >60 yearsCitation17.
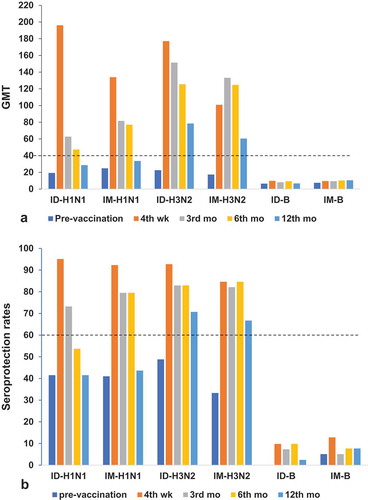
Table 6. Local and systemic side effects of the intradermal (ID, n = 36) and intramuscular (IM, n = 35) injection.
