Figures & data
Table 1. Comparison of the seroconversion rate of vaccine freeze exposure at −20°C and stored at 4°C. The seroconversion rate of vaccine freeze exposure at −20°C is lower than vaccine at 4°C. This result indicates that freezing and thawing caused a loss of potency for hepatitis E vaccines.
Figure 1. Freeze damage identification of HE vaccine indicated by shake test and A280 andA600. (A) The sedimentation and stratification were observed in freezing treated vaccine,while untreated vaccine was still in the form of a stable emulsion dispersed throughout an aqueous phase suspension. (B and C) Constant absorbance values were shown both in A280 and A600 for untreated vaccine. A280 for treated vaccine decreased from 1.100 to 0.366 in 10 min. A600 reflected the size and density of particles. Due to the particle aggregation, the initial A600 of untreated vaccine was 0.500, which is higher than 0.350 in untreated vaccine. And then the particles settled rapidly within 3 min and A600 value decreased to 0.100 at 10 min. Both absorbance values and shake tests could discriminate freezing exposure on rHE vaccine.
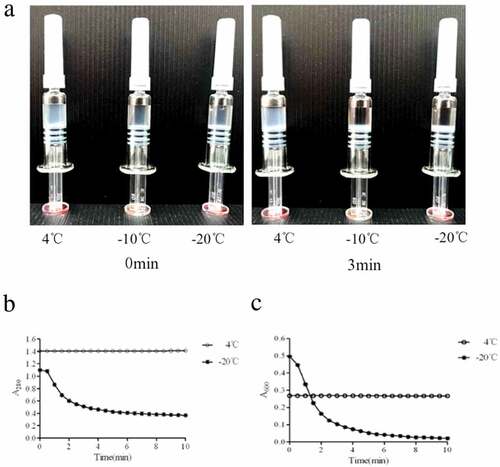
Figure 2. Different profile detected by Phase contrast microscopy. (A) The morphology of non-frozen samples showed fine-grain structure. (B) Freeze-damaged samples showed large conglomerates of massed precipitates with heterogeneous structures.
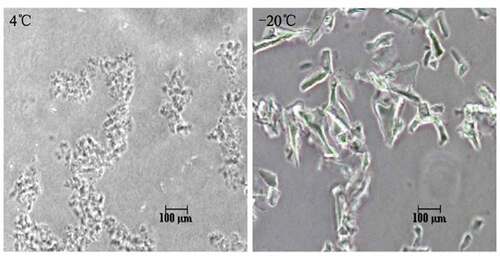
Figure 3. Comparison of dissolved antigen activity of vaccine before and after freeze exposure. (A) The antigen activity significantly decline in freezing damaged vaccine (p < 0.05), (B) Repeated freezing-thawing cycle significantly reduced the concentration of vaccine antigen(p < 0.01). (C and D) Consistent content of total protein were detected by BCA before and after freezing treatment. These results demonstrated the activity of antigen was decreased after freeze exposure. Error bars the standard deviation of three independent replicates.
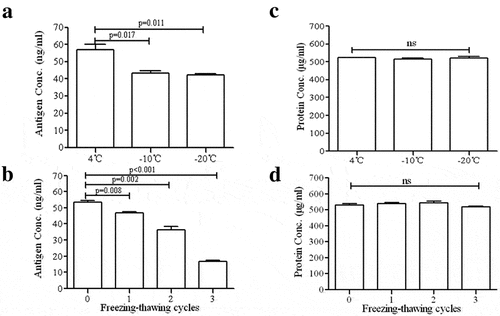
Figure 4. The epitope characteristics of the antigen reflected by 6 mAb. (A) The relative antigenicity to 6 mAb reduced significant in freezing treated samples (p < 0.001). (B) The antigenicity reduced gradually as the number of freeze-thaw cycles increases (p < 0.05). Error bars the standard deviation of three independent replicates.
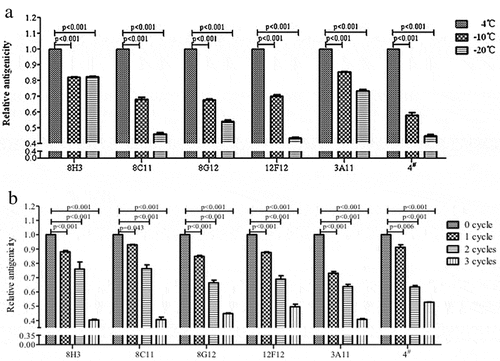
Table 2. The seroconversion rate of treated vaccine and untreated vaccine.
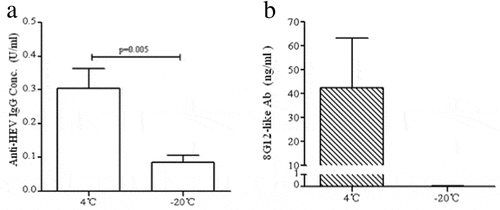
Figure 5. Quantification oftotal IgG and 8G12-like antibodies in mice serum vaccinated by 1.6 μg freezing treated or untreated vaccine. (A) Total IgG in serum vaccinated by treated vaccine decreased 3.6-fold than that in serum vaccinated by untreated vaccine (p = 0.005) (B) Specific neutralizing antibody 8G12-like antibody couldn’t be detected in frozen vaccine immunity serum, but the mean level of 8G12-like antibody in control group was 42.45 ng/ml.