Figures & data
Figure 1. Overview of human N- and O-glycosylation in the Golgi apparatus. On the left side, the synthesis of a human glycoprotein with several relevant complex-type N-glycans is shown. In the cis Golgi, mannosidase I (ManI) activity leads to a Man5GlcNAc2 that can be further modified in the medial Golgi. N-acetylglucosaminyltransferase I (GnTI) activity commits the glycan to the complex or hybrid type. Mannosidase II (ManII) activity, followed by several N-acetylglucosaminyltransferases then further commits the glycan to the complex type. If only N-acetylglucosaminyltransferases II (GnTII) acts on it, the result is a biantennary complex type N-glycan. GnTIV and/or GnTV activity then generates different triantennary or a tetraantennary complex type glycan. Fucosyltransferase VIII (FucTVIII) can act on any complex or hybrid type glycan to add a core α-1,6-fucose in the medial Golgi. Afterward, in the trans Golgi, galactosyltransferases (GalT), fucosyltransferases (FucT), sialyltransferases (SiaT) or a combination of GnTs and GalTs synthesize different capping moieties (sialylation, poly-LacNAc repeats, Lewis antigens) on N-glycans
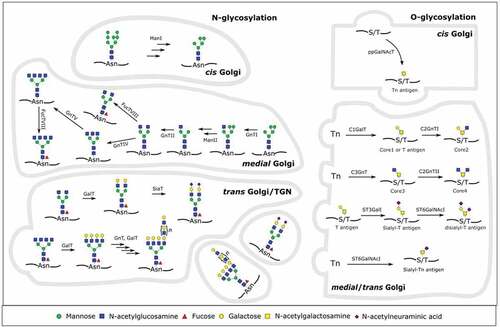
Table 1. Role of T cell glycosylation during T cell development, activation and signaling
Table 2. Role of glycan binding proteins in T cell cancer immunity
Table 3. Examples of glycan-engineering and its implications in immune therapy
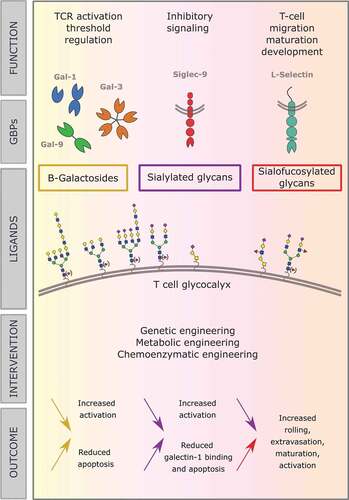
Figure 2. In this review, we discuss the role of different types of glycans decorating the surface of T cells, and their binding to GBPs and resulting functions. Central in the figure, several mammalian glycoforms that can occur on the T cell surface are shown. Above them, the GBPs that bind to them and the effects they have on T cells are outlined and below them we elaborate on the effects of therapeutic interventions on the glycan composition. The color of the arrows relates to the glycoforms, as indicated centrally in the figure, and the direction indicates decreased or increased expression of those glycoforms. The text next to the arrow then explains the effects on the T cell, resulting from the indicated changes in the glycoforms