Figures & data
Table 1. Baseline demographics (all participants).
Table 2. Proportions of participants achieving hSBA titers ≥1:8 at baseline (Day 0) and at Day 30 post-vaccination against serogroups A, C, W, and Y by vaccine group (PPAS).
Figure 1. Proportion of participants with an hSBA vaccine seroresponse* at Day 30 (PPAS) *Vaccine seroresponse is defined as a post-vaccination titer ≥1:8 if baseline titer is <1:8 at baseline or a ≥4-fold increase if baseline titer is ≥1:8.
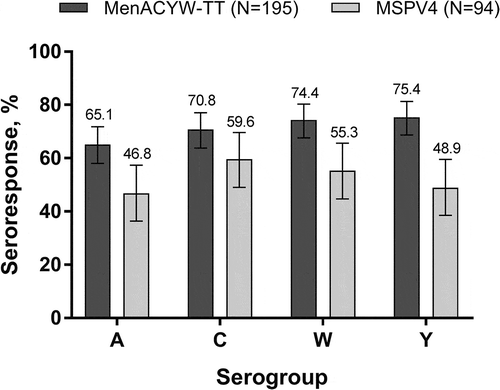
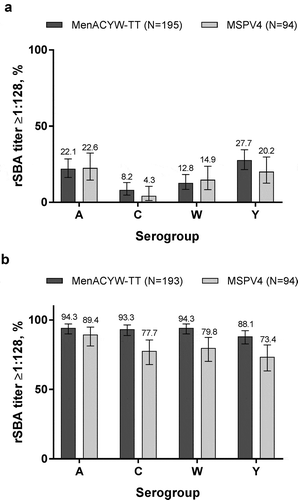
Table 3. Geometric mean antibody titers against serogroups A, C, W, and Y at baseline (Day 0) and Day 30 post-vaccination, as assessed by hSBA and rSBA (PPAS).
Table 4. Summary of safety data from Day 0 to Day 30 post-vaccination with MenACYW-TT or MPSV4 (safety analysis set).